Pulmonary matrix-derived hydrogels from patients with idiopathic pulmonary fibrosis induce a proinflammatory state in lung fibroblasts in vitro
- PMID: 38985514
- PMCID: PMC11321034
- DOI: 10.1091/mbc.E23-11-0428
Pulmonary matrix-derived hydrogels from patients with idiopathic pulmonary fibrosis induce a proinflammatory state in lung fibroblasts in vitro
Abstract
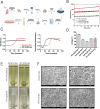
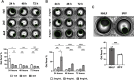
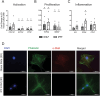
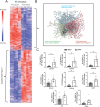
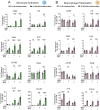
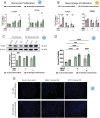
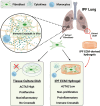
Idiopathic pulmonary fibrosis (IPF), one of the most common forms of interstitial lung disease, is a poorly understood, chronic, and often fatal fibroproliferative condition with only two FDA-approved medications. Understanding the pathobiology of the fibroblast in IPF is critical to evaluating and discovering novel therapeutics. Using a decellularized lung matrix derived from patients with IPF, we generate three-dimensional hydrogels as in vitro models of lung physiology and characterize the phenotype of fibroblasts seeded into the hydrogels. When cultured in IPF extracellular matrix hydrogels, IPF fibroblasts display differential contractility compared with their normal counterparts, lose the classical myofibroblast marker α-smooth muscle actin, and increase expression of proinflammatory cytokines compared with fibroblasts seeded two-dimensionally on tissue culture dishes. We validate this proinflammatory state in fibroblast-conditioned media studies with monocytes and monocyte-derived macrophages. These findings add to a growing understanding of the lung microenvironment effect on fibroblast phenotypes, shed light on the potential role of fibroblasts as immune signaling hubs during lung fibrosis, and suggest intervention in fibroblast-immune cell cross-talk as a possible novel therapeutic avenue.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

