RBCs regulate platelet function and hemostasis under shear conditions through biophysical and biochemical means
- PMID: 38985835
- PMCID: PMC11830970
- DOI: 10.1182/blood.2024023887
RBCs regulate platelet function and hemostasis under shear conditions through biophysical and biochemical means
Abstract
Red blood cells (RBCs) have been hypothesized to support hemostasis by facilitating platelet margination and releasing platelet-activating factors such as adenosine 5'-diphosphate (ADP). Significant knowledge gaps remain regarding how RBCs influence platelet function, especially in (patho)physiologically relevant hemodynamic conditions. Here, we present results showing how RBCs affect platelet function and hemostasis in conditions of anemia, thrombocytopenia, and pancytopenia and how the biochemical and biophysical properties of RBCs regulate platelet function at the blood and vessel wall interface and in the fluid phase under flow conditions. We found that RBCs promoted platelet deposition to collagen under flow conditions in moderate (50 × 103/μL) but not severe (10 × 103/μL) thrombocytopenia in vitro. Reduction in hematocrit by 45% increased bleeding in mice with hemolytic anemia. In contrast, bleeding diathesis was observed in mice with a 90% but not with a 60% reduction in platelet counts. RBC transfusion improved hemostasis by enhancing fibrin clot formation at the site of vascular injury in mice with severe pancytopenia induced by total body irradiation. Altering membrane deformability changed the ability of RBCs to promote shear-induced platelet aggregation. RBC-derived ADP contributed to platelet activation and aggregation in vitro under pathologically high shear stresses, as observed in patients supported by left ventricular assist devices. These findings demonstrate that RBCs support platelet function and hemostasis through multiple mechanisms, both at the blood and vessel wall interface and in the fluidic phase of circulation.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: T.B.G. claims no relevant conflicts of interest but reports being a member of the medical advisory board for Platelet Disorder Support Association; receiving consultation fees from Sanofi Corporation, Sobi Corporation, Alpine Immune Science: Bioproducts Laboratory, Cellphire Corporation, Novartis, and Amgen; is a member of the data safety monitoring board of Palisade Bio and Novartis; and receives honoraria from Amgen Inc and Sobi Corporation. The remaining authors declare no competing financial interests.
Figures

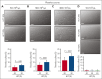

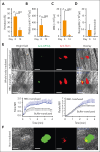


Comment in
-
Red blood cells: more than bags of hemoglobin.Blood. 2024 Oct 3;144(14):1467-1469. doi: 10.1182/blood.2024026048. Blood. 2024. PMID: 39361301 No abstract available.
References
-
- Livio M, Marchesi D, Remuzzi G, Mecca G, Remuzzi G, de Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;2(8306):1013–1015. - PubMed
-
- Valeri CR, Cassidy G, Pivacek LE, et al. Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion (Paris) 2001;41(8):977–983. - PubMed
-
- Crowley JP, Metzger JB, Robert Valeri C. The volume of blood shed during the bleeding time correlates with the peripheral venous hematocrit. Am J Clin Pathol. 1997;108(5):579–584. - PubMed
-
- Ho CH. The hemostatic effect of packed red cell transfusion in patients with anemia. Transfusion (Paris) 1998;38(11-12):1011–1014. - PubMed
-
- Hung Ho C. Increase of red blood cells can shorten the bleeding time in patients with iron deficiency anemia. Blood. 1998;91(3):1094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

