Sex differences in neural projections of fear memory processing in mice and humans
- PMID: 38985873
- PMCID: PMC11235172
- DOI: 10.1126/sciadv.adk3365
Sex differences in neural projections of fear memory processing in mice and humans
Abstract
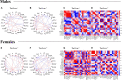
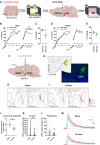
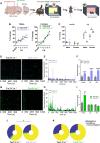
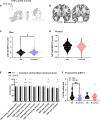
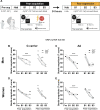
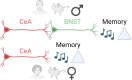
It remains unexplored in the field of fear memory whether functional neuronal connectivity between two brain areas is necessary for one sex but not the other. Here, we show that chemogenetic silencing of centromedial (CeM)-Tac2 fibers in the lateral posterior BNST (BNSTpl) decreased fear memory consolidation in male mice but not females. Optogenetic excitation of CeM-Tac2 fibers in the BNSTpl exhibited enhanced inhibitory postsynaptic currents in males compared to females. In vivo calcium imaging analysis revealed a sex-dimorphic fear memory engram in the BNSTpl. Furthermore, in humans, the single-nucleotide polymorphism (SNP) in the Tac2 receptor (rs2765) (TAC3R) decreased CeM-BNST connectivity in a fear task, impaired fear memory consolidation, and increased the expression of the TAC3R mRNA in AA-carrier men but not in women. These sex differences in critical neuronal circuits underlying fear memory formation may be relevant to human neuropsychiatric disorders with fear memory alterations such as posttraumatic stress disorder.
Figures

References
-
- Kandel E. R., The molecular biology of memory storage: A dialogue between genes and synapses. Science 294, 1030–1038 (2001). - PubMed
-
- Kandel E. R., Dudai Y., Mayford M. R., The molecular and systems biology of memory. Cell 157, 163–186 (2014). - PubMed
-
- Flores Á., Fullana M. À., Soriano-Mas C., Andero R., Lost in translation: How to upgrade fear memory research. Mol. Psychiatry 23, 2122–2132 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

