SLC10A5 deficiency causes hypercholanemia
- PMID: 38986003
- PMCID: PMC11737122
- DOI: 10.1097/HEP.0000000000000994
SLC10A5 deficiency causes hypercholanemia
Abstract
Background and aims: Solute Carrier Family 10 Member 5 (SLC10A5) is a member of SLC10, comprising transporters of bile acids, steroidal hormones, and other substrates, but its function remains unclear. The aim of the current investigation was to clarify its function in the metabolism of bile acid and hypercholanemia.
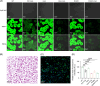
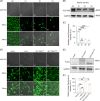
Approach and results: Whole-exome sequencing and Sanger sequencing were used to identify and confirm the variant in the subjects of hypercholanemia. CRISPR/Cas9-mediated genome engineering was used to establish the knockout and point mutation mice. Primary mouse hepatocytes were isolated, and cell lines were cultured. SLC10A5 was silenced by siRNA and overexpressed by wild-type and mutant plasmids. The fluorescent bile acid derivative was used for the bile acid uptake assay. Bile acids were assessed with ultra-performance liquid chromatography tandem mass spectrometry. A heterozygous variant SLC10A5 : c.994_995del (p.D332X) was identified in subjects with elevated total bile acid or altered bile acid profiles. Bile acids were increased in the serum and liver of knockout and point mutation mice. The expressions of FXR and SHP, regulators involved in the negative feedback of bile acid synthesis, were downregulated, while the bile acid synthesis genes CYP7A1 and CYP8B1 were upregulated in both gene-edited mice. Both the wild and mutant SLC10A5 proteins were localized on the plasma membrane. Knockdown, knockout, or targeted mutation of SLC10A5 led to the inhibition of bile acid uptake by cell lines and primary mouse hepatocytes.
Conclusion: SLC10A5 is involved in the uptake of bile acid, and its deficiency causes hypercholanemia.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts to report.
Figures





Similar articles
-
The novel putative bile acid transporter SLC10A5 is highly expressed in liver and kidney.Biochem Biophys Res Commun. 2007 Sep 14;361(1):26-32. doi: 10.1016/j.bbrc.2007.06.160. Epub 2007 Jul 10. Biochem Biophys Res Commun. 2007. PMID: 17632081
-
Increased sulfation of bile acids in mice and human subjects with sodium taurocholate cotransporting polypeptide deficiency.J Biol Chem. 2019 Aug 2;294(31):11853-11862. doi: 10.1074/jbc.RA118.007179. Epub 2019 Jun 14. J Biol Chem. 2019. PMID: 31201272 Free PMC article.
-
The solute carrier family 10 (SLC10): beyond bile acid transport.Mol Aspects Med. 2013 Apr-Jun;34(2-3):252-69. doi: 10.1016/j.mam.2012.07.004. Mol Aspects Med. 2013. PMID: 23506869 Free PMC article. Review.
-
Clinical and molecular characterization of four patients with NTCP deficiency from two unrelated families harboring the novel SLC10A1 variant c.595A>C (p.Ser199Arg).Mol Med Rep. 2019 Dec;20(6):4915-4924. doi: 10.3892/mmr.2019.10763. Epub 2019 Oct 23. Mol Med Rep. 2019. PMID: 31661128 Free PMC article.
-
The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships.Naunyn Schmiedebergs Arch Pharmacol. 2006 Mar;372(6):413-31. doi: 10.1007/s00210-006-0043-8. Epub 2006 Mar 16. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16541252 Review.
References
-
- Boyer JL, Soroka CJ. Bile formation and secretion: An update. J Hepatol. 2021;75:190–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

