Quantitative Integrative Survival Prediction in Multiple Myeloma Patients Treated With Bortezomib-Based Induction, High-Dose Therapy and Autologous Stem Cell Transplantation
- PMID: 38986047
- PMCID: PMC11371111
- DOI: 10.1200/PO.23.00613
Quantitative Integrative Survival Prediction in Multiple Myeloma Patients Treated With Bortezomib-Based Induction, High-Dose Therapy and Autologous Stem Cell Transplantation
Abstract
Purpose: Given the high heterogeneity in survival for patients with multiple myeloma, it would be clinically useful to quantitatively predict the individual survival instead of attributing patients to two to four risk groups as in current models, for example, revised International Staging System (R-ISS), R2-ISS, or Mayo-2022-score.
Patients and methods: Our aim was to develop a quantitative prediction tool for individual patient's 3-/5-year overall survival (OS) probability. We integrated established clinical and molecular risk factors into a comprehensive prognostic model and evaluated and validated its risk discrimination capabilities versus R-ISS, R2-ISS, and Mayo-2022-score.
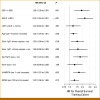
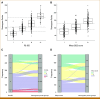
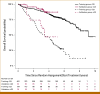
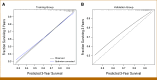
Results: A nomogram for estimating OS probabilities was built on the basis of a Cox regression model. It allows one to translate the individual risk profile of a patient into 3-/5-year OS probabilities by attributing points to each prognostic factor and summing up all points. The nomogram was externally validated regarding discrimination and calibration. There was no obvious bias or overfitting of the prognostic index on the validation cohort. Resampling-based and external evaluation showed good calibration. The c-index of the model was similar on the training (0.76) and validation cohort (0.75) and significantly higher than for the R-ISS (P < .001) or R2-ISS (P < .01).
Conclusion: In summary, we developed and validated individual quantitative nomogram-based OS prediction. Continuous risk assessment integrating molecular prognostic factors is superior to R-ISS, R2-ISS, or Mayo-2022-score alone.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Kyle RA, Rajkumar SV: Multiple myeloma. N Engl J Med 351:1860-1873, 2004 - PubMed
-
- Goldschmidt H, Lokhorst HM, Mai EK, et al. : Bortezomib before and after high-dose therapy in myeloma: Long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 32:383-390, 2018 - PubMed
-
- Dimopoulos MA, Terpos E, Boccadoro M, et al. : Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol 22:801-812, 2021 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

