Human effector CD8+ T cells with an activated and exhausted-like phenotype control tumour growth in vivo in a humanized tumour model
- PMID: 38986249
- PMCID: PMC11296066
- DOI: 10.1016/j.ebiom.2024.105240
Human effector CD8+ T cells with an activated and exhausted-like phenotype control tumour growth in vivo in a humanized tumour model
Abstract
Background: Humanized tumour models could be particularly valuable for cancer immunotherapy research, as they may better reflect human-specific aspects of the interfaces between tumour and immune system of human cancer. However, endogenous antitumour immunity in humanized models is still largely undefined.
Methods: We established an autologous humanized mouse tumour model by using NSG mice reconstituted with human immune cells from hematopoietic progenitors and tumours generated from transformed autologous human B cells. We demonstrate growth of solid lymphoid tumours after subcutaneous implantation, infiltration by endogenous human immune cells and immunocompetence of the model.
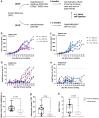
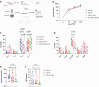
Findings: We found human T cell subsets described in human cancer, including progenitor exhausted (Tpex), terminally exhausted (Tex-term) and tissue-resident (TRM) cells in tumour-bearing humanized mice with accumulation of Tex-term and TRM in the tumour. In addition, we identified tumour-reactive CD8+ T cells through expression of CD137. This subpopulation of de novo arising human CD137+ CD8+ T cells displayed a highly proliferative, fully activated effector and exhausted-like phenotype with enhanced expression of activation and exhaustion markers like PD-1, CD39, CD160, TIM-3, TIGIT and TOX, the senescence marker CD57 (B3GAT1) and cytolytic effector molecules such as PRF1, GZMH and NKG7. Moreover, these CD137+ CD8+ T cells exhibited tumour-specific clonal expansion and presented signature overlap with tumour-reactive CD8+ T cells described in human cancer. We demonstrate superior anticancer activity of this activated and exhausted-like human CD8+ T cell subset by adoptive transfer experiments using recipients bearing autologous human tumours. Mice adoptively transferred with CD137+ CD8+ T cells showed reduced tumour growth and higher CD8+ T cell tumour infiltration, correlating with control of human tumours.
Interpretation: We established an immunocompetent humanized tumour model, providing a tool for immunotherapy research and defined effective anticancer activity of human effector CD8+ T cells with an activated and exhausted-like phenotype, supporting clinical exploration of such cells in adoptive T cell therapies.
Funding: Swiss Cancer Research foundation.
Keywords: Adoptive cell therapy; Exhaustion; Human cancer immunology; Humanized cancer models; Phenotypic markers; T cells.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures




Similar articles
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
-
Resident memory T cells, critical components in tumor immunology.J Immunother Cancer. 2018 Sep 4;6(1):87. doi: 10.1186/s40425-018-0399-6. J Immunother Cancer. 2018. PMID: 30180905 Free PMC article. Review.
-
Enhanced Phenotype Definition for Precision Isolation of Precursor Exhausted Tumor-Infiltrating CD8 T Cells.Front Immunol. 2020 Feb 27;11:340. doi: 10.3389/fimmu.2020.00340. eCollection 2020. Front Immunol. 2020. PMID: 32174925 Free PMC article.
-
Activin-A impedes the establishment of CD4+ T cell exhaustion and enhances anti-tumor immunity in the lung.J Exp Clin Cancer Res. 2021 Sep 21;40(1):295. doi: 10.1186/s13046-021-02092-5. J Exp Clin Cancer Res. 2021. PMID: 34548096 Free PMC article.
-
NR4A transcription factors limit CAR T cell function in solid tumours.Nature. 2019 Mar;567(7749):530-534. doi: 10.1038/s41586-019-0985-x. Epub 2019 Feb 27. Nature. 2019. PMID: 30814732 Free PMC article.
References
-
- Simoni Y., Becht E., Fehlings M., et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. - PubMed
-
- Scheper W., Kelderman S., Fanchi L.F., et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. 2019;25(1):89–94. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

