Antibody modulation of B cell responses-Incorporating positive and negative feedback
- PMID: 38986442
- PMCID: PMC11257158
- DOI: 10.1016/j.immuni.2024.06.009
Antibody modulation of B cell responses-Incorporating positive and negative feedback
Abstract
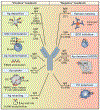
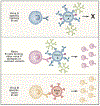
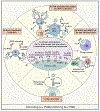
Antibodies are powerful modulators of ongoing and future B cell responses. While the concept of antibody feedback has been appreciated for over a century, the topic has seen a surge in interest due to the evidence that the broadening of antibody responses to SARS-CoV-2 after a third mRNA vaccination is a consequence of antibody feedback. Moreover, the discovery that slow antigen delivery can lead to more robust humoral immunity has put a spotlight on the capacity for early antibodies to augment B cell responses. Here, we review the mechanisms whereby antibody feedback shapes B cell responses, integrating findings in humans and in mouse models. We consider the major influence of epitope masking and the diverse actions of complement and Fc receptors and provide a framework for conceptualizing the ways antigen-specific antibodies may influence B cell responses to any form of antigen, in conditions as diverse as infectious disease, autoimmunity, and cancer.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors make the following disclosures: J.G..C. is a member of the Scientific Advisory Board of BeBio Pharma and consults for Lycia Therapeutics and DrenBio Inc. P.C.W. is a member of the Scientific Advisory boards of Evozyne, Inc. and Invivyd, Inc.
Figures

References
-
- Heyman B. (2000). Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol 18, 709–737. - PubMed
-
- Tew JG, Wu J, Qin D, Helm S, Burton GF, and Szakal AK (1997). Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev 156, 39–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

