Genomic, immunologic, and prognostic associations of TROP2 (TACSTD2) expression in solid tumors
- PMID: 38986529
- PMCID: PMC11546728
- DOI: 10.1093/oncolo/oyae168
Genomic, immunologic, and prognostic associations of TROP2 (TACSTD2) expression in solid tumors
Abstract
Background: TROP2 (TACSTD2) expression is associated with decreased overall survival (OS) in some solid tumors, and the TROP2-targeting antibody-drug conjugate (ADC) sacituzumab govitecan has been approved in breast and urothelial carcinomas. We aimed to explore the multi-omic landscape associated with TACSTD2 gene expression in various solid tumors to identify patients most likely to benefit from this approach.
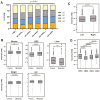
Methods: Breast (N = 11 246), colorectal (N = 15 425), hepatocellular (N = 433), pancreatic (N = 5488), and urothelial (N = 4125) tumors were stratified into quartiles by TACSTD2 gene expression, analyzed by next-generation DNA sequencing, whole transcriptome sequencing, and immunohistochemistry at Caris Life Sciences (Phoenix, AZ). Survival data were obtained from insurance claims, and Kaplan-Meier estimates were calculated for molecularly defined cohorts.
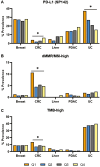
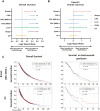
Results: Several pathogenic mutations were associated with TACSTD2-high tumors, including TP53 in breast, colorectal (CRC), pancreatic, and hepatocellular cancers; KRAS in pancreatic and CRC cancers; ARID1A and FGFR3 in urothelial cancer; and CTNNB1 in hepatocellular cancer. TACSTD2-low breast tumors were enriched for copy number amplifications in CCND1 and FGF/R family member genes. TACSTD2 high was generally associated with more immune cell infiltration and greater T-cell inflammation scores. Patients with TACSTD2-high breast, CRC, and pancreatic cancers demonstrated a significantly shorter OS than TACSTD2-low tumors. This was restricted to CRC with microsatellite stable tumors and patients with pancreatic cancer with KRAS-mutant tumors. Patients with breast cancer with TACSTD2-high tumors also experienced significantly worse OS following immune checkpoint inhibitors.
Conclusions: TACSTD2 expression is associated with key driver alterations and a more active immune microenvironment, suggesting possible combinatorial strategies with TROP2-targeting ADCs plus immunotherapy in various solid tumors.
Keywords: TROP2; precision oncology; targeted therapy; tumor genetics.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
E.S.A. reports grants and personal fees from Janssen, Sanofi, Bayer, Bristol Myers Squibb, Curium, Merck, Pfizer, AstraZeneca, and Clovis; personal fees from Astellas, Amgen, Blue Earth, Exact Sciences, Invitae, Eli Lilly, and Foundation Medicine; grants from Novartis, Celgene, and Orion; and has a patent for an AR-V7 biomarker technology that has been licensed to Qiagen. E.L. reports support from the University of Minnesota Clinical Center for the Study of Pancreatic Disease, part of The Chronic Pancreatitis Diabetes Pancreatic Cancer research (CPDPC) consortium funded by the NIDDK (5U01DK126300-03), and research grants from the American Cancer Society (RSG-22-022-01-CDP) 2022-2026; The Randy Shaver Cancer Research and Community Fund; compensation for scientific review of proposed printed content, Elsevier Publishing and Johns Hopkins Press; Institutional Principal Investigator for clinical trials sponsored by Celgene, Novocure, Ltd, Intima Bioscience, Inc., the National Cancer Institute, and University of Minnesota membership in the Caris Life Sciences Precision Oncology Alliance (no financial compensation).
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

