Lee Silverman voice treatment versus NHS speech and language therapy versus control for dysarthria in people with Parkinson's disease (PD COMM): pragmatic, UK based, multicentre, three arm, parallel group, unblinded, randomised controlled trial
- PMID: 38986549
- PMCID: PMC11232530
- DOI: 10.1136/bmj-2023-078341
Lee Silverman voice treatment versus NHS speech and language therapy versus control for dysarthria in people with Parkinson's disease (PD COMM): pragmatic, UK based, multicentre, three arm, parallel group, unblinded, randomised controlled trial
Abstract
Objectives: To assess the clinical effectiveness of two speech and language therapy approaches versus no speech and language therapy for dysarthria in people with Parkinson's disease.
Design: Pragmatic, UK based, multicentre, three arm, parallel group, unblinded, randomised controlled trial.
Setting: The speech and language therapy interventions were delivered in outpatient or home settings between 26 September 2016 and 16 March 2020.
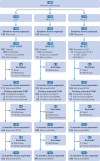
Participants: 388 people with Parkinson's disease and dysarthria.
Interventions: Participants were randomly assigned to one of three groups (1:1:1): 130 to Lee Silverman voice treatment (LSVT LOUD), 129 to NHS speech and language therapy, and 129 to no speech and language therapy. LSVT LOUD consisted of four, face-to-face or remote, 50 min sessions each week delivered over four weeks. Home based practice activities were set for up to 5-10 mins daily on treatment days and 15 mins twice daily on non-treatment days. Dosage for the NHS speech and language therapy was determined by the local therapist in response to the participants' needs (estimated from prior research that NHS speech and language therapy participants would receive an average of one session per week over six to eight weeks). Local practices for NHS speech and language therapy were accepted, except for those within the LSVT LOUD protocol. Analyses were based on the intention to treat principle.
Main outcome measures: The primary outcome was total score at three months of self-reported voice handicap index.
Results: People who received LSVT LOUD reported lower voice handicap index scores at three months after randomisation than those who did not receive speech and language therapy (-8.0 points (99% confidence interval -13.3 to -2.6); P<0.001). No evidence suggests a difference in voice handicap index scores between NHS speech and language therapy and no speech and language therapy (1.7 points (-3.8 to 7.1); P=0.43). Patients in the LSVT LOUD group also reported lower voice handicap index scores than did those randomised to NHS speech and language therapy (-9.6 points (-14.9 to -4.4); P<0.001). 93 adverse events (predominately vocal strain) were reported in the LSVT LOUD group, 46 in the NHS speech and language therapy group, and none in the no speech and language therapy group. No serious adverse events were recorded.
Conclusions: LSVT LOUD was more effective at reducing the participant reported impact of voice problems than was no speech and language therapy and NHS speech and language therapy. NHS speech and language therapy showed no evidence of benefit compared with no speech and language therapy.
Trial registration: ISRCTN registry ISRCTN12421382.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work or describe if any; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Speech therapy for people with Parkinson's disease.BMJ. 2024 Jul 10;386:q1254. doi: 10.1136/bmj.q1254. BMJ. 2024. PMID: 38986539 No abstract available.
References
-
- Duffy J. In: Heights M, ed. Motor speech disorders: substrates, differential diagnosis, and management. Fourth. Elsevier, 2019.
-
- Heberlein IVP. The influence of speech disturbances on quality of life and coping strategies on Parkinson’s disease patients. Forum Logopadie, 2005: 26-31.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical