Itaconate is a metabolic regulator of bone formation in homeostasis and arthritis
- PMID: 38986577
- PMCID: PMC11503170
- DOI: 10.1136/ard-2023-224898
Itaconate is a metabolic regulator of bone formation in homeostasis and arthritis
Abstract
Objectives: Bone remodelling is a highly dynamic process dependent on the precise coordination of osteoblasts and haematopoietic-cell derived osteoclasts. Changes in core metabolic pathways during osteoclastogenesis, however, are largely unexplored and it is unknown whether and how these processes are involved in bone homeostasis.
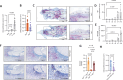
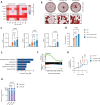
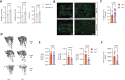
Methods: We metabolically and transcriptionally profiled cells during osteoclast and osteoblast generation. Individual gene expression was characterised by quantitative PCR and western blot. Osteoblast function was assessed by Alizarin red staining. immunoresponsive gene 1 (Irg1)-deficient mice were used in various inflammatory or non-inflammatory models of bone loss. Tissue gene expression was analysed by RNA in situ hybridisation.
Results: We show that during differentiation preosteoclasts rearrange their tricarboxylic acid cycle, a process crucially depending on both glucose and glutamine. This rearrangement is characterised by the induction of Irg1 and production of itaconate, which accumulates intracellularly and extracellularly. While the IRG1-itaconate axis is dispensable for osteoclast generation in vitro and in vivo, we demonstrate that itaconate stimulates osteoblasts by accelerating osteogenic differentiation in both human and murine cells. This enhanced osteogenic differentiation is accompanied by reduced proliferation and altered metabolism. Additionally, supplementation of itaconate increases bone formation by boosting osteoblast activity in mice. Conversely, Irg1-deficient mice exhibit decreased bone mass and have reduced osteoproliferative lesions in experimental arthritis.
Conclusion: In summary, we identify itaconate, generated as a result of the metabolic rewiring during osteoclast differentiation, as a previously unrecognised regulator of osteoblasts.
Keywords: arthritis, experimental; bone density; spondylitis, ankylosing.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

