DEAD box RNA helicases are pervasive protein kinase interactors and activators
- PMID: 38986579
- PMCID: PMC11293542
- DOI: 10.1101/gr.278264.123
DEAD box RNA helicases are pervasive protein kinase interactors and activators
Abstract
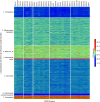
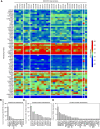
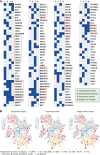
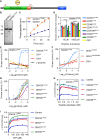
DEAD box (DDX) RNA helicases are a large family of ATPases, many of which have unknown functions. There is emerging evidence that besides their role in RNA biology, DDX proteins may stimulate protein kinases. To investigate if protein kinase-DDX interaction is a more widespread phenomenon, we conducted three orthogonal large-scale screens, including proteomics analysis with 32 RNA helicases, protein array profiling, and kinome-wide in vitro kinase assays. We retrieved Ser/Thr protein kinases as prominent interactors of RNA helicases and report hundreds of binary interactions. We identified members of ten protein kinase families, which bind to, and are stimulated by, DDX proteins, including CDK, CK1, CK2, DYRK, MARK, NEK, PRKC, SRPK, STE7/MAP2K, and STE20/PAK family members. We identified MARK1 in all screens and validated that DDX proteins accelerate the MARK1 catalytic rate. These findings indicate pervasive interactions between protein kinases and DEAD box RNA helicases, and provide a rich resource to explore their regulatory relationships.
© 2024 Hirth et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Bailey T, Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology, pp. 28–36. AAAI Press, Cambridge, MA. - PubMed
-
- Barouch-Bentov R, Che J, Lee CC, Yang Y, Herman A, Jia Y, Velentza A, Watson J, Sternberg L, Kim S, et al. 2009. A conserved salt bridge in the G loop of multiple protein kinases is important for catalysis and for in vivo Lyn function. Mol Cell 33: 43–52. 10.1016/j.molcel.2008.12.024 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
