Trends and outcomes of fresh and frozen donor oocyte cycles in the United States
- PMID: 38986758
- PMCID: PMC11560672
- DOI: 10.1016/j.fertnstert.2024.07.004
Trends and outcomes of fresh and frozen donor oocyte cycles in the United States
Abstract
Objective: To examine trends, characteristics, and outcomes of donor oocyte embryo transfer cycles by original oocyte and resultant embryo state and determine whether oocyte state (fresh or frozen) is differentially associated with clinical pregnancy, live birth, and term, healthy birthweight neonates among singleton live births.
Design: Retrospective cohort study.
Setting: National study.
Patient(s): Patients undergoing donor oocyte embryo transfer cycles in the United States reporting to the National Assisted Reproductive Technology Surveillance System from 2013 to 2020. INTERVENTION(S): Original donor oocyte and resultant embryo state (fresh or frozen).
Main outcome measure(s): Annual numbers and proportions of total donor oocyte embryo transfer cycles stratified by oocyte and embryo state and single embryo transfer cycles resulting in the live birth of term (≥37 weeks gestation), healthy birthweight (≥2,500 g) singletons during 2013-2020. Rates of live birth and term, healthy birthweight neonates among singleton live births for 2018-2020 are also reported. Relative risks examine associations between donor oocyte state and live birth and term, healthy birthweight neonates among singleton live births resulting from donor oocyte embryo transfer cycles.
Result(s): From 2013 to 2020, there were 135,085 donor oocyte embryo transfer cycles, of which the proportions increased for frozen embryos (42.3%-76.6%), fresh embryos using frozen donor oocytes (19.9%-68.3%) and single embryo transfers (36.4%-85.5%). During 2018-2020, there were 48,679 donor oocyte embryo transfer cycles. Rates of live birth were lower with frozen compared with fresh donor oocytes for both fresh (46.2%, 55.9%; adjusted relative risk [aRR], 0.83; 95% confidence interval [CI], 0.79-0.87) and frozen (41.3%, 45.8%; aRR, 0.94; 95% CI, 0.91-0.98) embryo transfer cycles. Among singleton live births, rates of delivering a term, healthy birth weight neonate were similar for frozen compared with fresh donor oocyte transfer cycles among fresh (77.3%, 77.2%; aRR, 1.01; 95% CI, 0.98-1.03) and frozen (75.6%, 75.1%; aRR, 1.02; 95% CI, 0.99-1.04) embryos.
Conclusion(s): In this national study of donor oocyte embryo transfer cycles, frozen embryo transfers, fresh embryo transfers using frozen oocytes, and single embryo transfers increased. Although frozen compared with fresh oocytes were associated with a slightly reduced rate of live birth, rates of term, healthy birthweight neonates among singleton live births were comparable between donor oocyte states.
Keywords: Donor oocyte; cryopreservation; frozen; live birth; pregnancy outcome.
Copyright © 2024 American Society for Reproductive Medicine. All rights reserved.
Conflict of interest statement
Declaration of Interests C.B.B. reports funding from Marianne Ruby, MD Award from the Department of Gynecology and Obstetrics, Emory University School of Medicine for a senior medical student to conduct research in an area that will advance knowledge and understanding of issues impacting women from menarche through menopause; and the Pacific Coast Reproductive Society Scholarship for part of this research to be presented at the Annual Pacific Coast Reproductive Society Conference in Indian Wells, California, March 22–26, 2023. C.E.D. has nothing to disclose. J.C.L. has nothing to disclose. D.M.K. has nothing to disclose. J.F.K. has nothing to disclose.
Figures
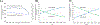
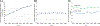
References
-
- Christianson MS, Bellver J. Innovations in assisted reproductive technologies: impact on contemporary donor egg practice and future advances. Fertil Steril 2018;110:994–1002. - PubMed
-
- Centers for Disease Control and Prevention. 2020 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2022.
-
- Centers for Disease Control and Prevention. Morbidity and mortality weekly report: Assisted Reproductive Technology Surveillance—2000. US Dept of Health and Human Services; 2003.
-
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021.
-
- Guidance regarding gamete and embryo donation. Fertil Steril 2021;115:1395–410. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

