Loss of Fic causes progressive neurodegeneration in a Drosophila model of hereditary spastic paraplegia
- PMID: 38986817
- PMCID: PMC11549967
- DOI: 10.1016/j.bbadis.2024.167348
Loss of Fic causes progressive neurodegeneration in a Drosophila model of hereditary spastic paraplegia
Abstract
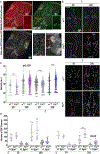
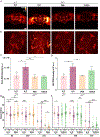
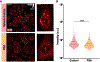
Hereditary Spastic Paraplegia (HSP) is a group of rare inherited disorders characterized by progressive weakness and spasticity of the legs. Recent newly discovered biallelic variants in the gene FICD were found in patients with a highly similar phenotype to early onset HSP. FICD encodes filamentation induced by cAMP domain protein. FICD is involved in the AMPylation and deAMPylation protein modifications of the endoplasmic reticulum (ER) chaperone BIP, a major constituent of the ER that regulates the unfolded protein response. Although several biochemical properties of FICD have been characterized, the neurological function of FICD and the pathological mechanism underlying HSP are unknown. We established a Drosophila model to gain mechanistic understanding of the function of FICD in HSP pathogenesis, and specifically the role of BIP in neuromuscular physiology. Our studies on Drosophila Fic null mutants uncovered that loss of Fic resulted in locomotor impairment and reduced levels of BIP in the motor neuron circuitry, as well as increased reactive oxygen species (ROS) in the ventral nerve cord of Fic null mutants. Finally, feeding Drosophila Fic null mutants with chemical chaperones PBA or TUDCA, or treatment of patient fibroblasts with PBA, reduced the ROS accumulation. The neuronal phenotypes of Fic null mutants recapitulate several clinical features of HSP patients and further reveal cellular patho-mechanisms. By modeling FICD in Drosophila, we provide potential targets for intervention for HSP, and advance fundamental biology that is important for understanding related rare and common neuromuscular diseases.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Authors declare that they have no competing interests.
Figures


References
-
- Martinello C, Panza E, & Orlacchio A, Hereditary spastic paraplegias proteome: common pathways and pathogenetic mechanisms. Expert Review of Proteomics, 2023. 20(7–9): p. 171–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

