Compartmentalized mitochondrial ferroptosis converges with optineurin-mediated mitophagy to impact airway epithelial cell phenotypes and asthma outcomes
- PMID: 38987265
- PMCID: PMC11237105
- DOI: 10.1038/s41467-024-50222-2
Compartmentalized mitochondrial ferroptosis converges with optineurin-mediated mitophagy to impact airway epithelial cell phenotypes and asthma outcomes
Abstract
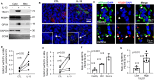
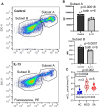
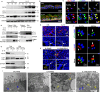
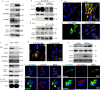
A stable mitochondrial pool is crucial for healthy cell function and survival. Altered redox biology can adversely affect mitochondria through induction of a variety of cell death and survival pathways, yet the understanding of mitochondria and their dysfunction in primary human cells and in specific disease states, including asthma, is modest. Ferroptosis is traditionally considered an iron dependent, hydroperoxy-phospholipid executed process, which induces cytosolic and mitochondrial damage to drive programmed cell death. However, in this report we identify a lipoxygenase orchestrated, compartmentally-targeted ferroptosis-associated peroxidation process which occurs in a subpopulation of dysfunctional mitochondria, without promoting cell death. Rather, this mitochondrial peroxidation process tightly couples with PTEN-induced kinase (PINK)-1(PINK1)-Parkin-Optineurin mediated mitophagy in an effort to preserve the pool of functional mitochondria and prevent cell death. These combined peroxidation processes lead to altered epithelial cell phenotypes and loss of ciliated cells which associate with worsened asthma severity. Ferroptosis-targeted interventions of this process could preserve healthy mitochondria, reverse cell phenotypic changes and improve disease outcomes.
© 2024. The Author(s).
Conflict of interest statement
Dr. Wenzel is principal investigator on an investigator initiated/single center study of mucociliary clearance and the IL-4R antibody dupilumab in asthma patients funded by Regeneron. No other authors declare any conflicts.
Figures

References
MeSH terms
Substances
Grants and funding
- AI145406/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P01 AI106684/AI/NIAID NIH HHS/United States
- R01 HL153058/HL/NHLBI NIH HHS/United States
- HL153058/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P01 AI106684-06A1/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

