Bioengineered niches that recreate physiological extracellular matrix organisation to support long-term haematopoietic stem cells
- PMID: 38987295
- PMCID: PMC11237034
- DOI: 10.1038/s41467-024-50054-0
Bioengineered niches that recreate physiological extracellular matrix organisation to support long-term haematopoietic stem cells
Abstract
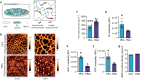
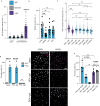
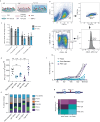
Long-term reconstituting haematopoietic stem cells (LT-HSCs) are used to treat blood disorders via stem cell transplantation. The very low abundance of LT-HSCs and their rapid differentiation during in vitro culture hinders their clinical utility. Previous developments using stromal feeder layers, defined media cocktails, and bioengineering have enabled HSC expansion in culture, but of mostly short-term HSCs and progenitor populations at the expense of naive LT-HSCs. Here, we report the creation of a bioengineered LT-HSC maintenance niche that recreates physiological extracellular matrix organisation, using soft collagen type-I hydrogels to drive nestin expression in perivascular stromal cells (PerSCs). We demonstrate that nestin, which is expressed by HSC-supportive bone marrow stromal cells, is cytoprotective and, via regulation of metabolism, is important for HIF-1α expression in PerSCs. When CD34+ve HSCs were added to the bioengineered niches comprising nestin/HIF-1α expressing PerSCs, LT-HSC numbers were maintained with normal clonal and in vivo reconstitution potential, without media supplementation. We provide proof-of-concept that our bioengineered niches can support the survival of CRISPR edited HSCs. Successful editing of LT-HSCs ex vivo can have potential impact on the treatment of blood disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- State of the Registry 2020–2021. In: Anthony Nolan NHS Stem Cell Registry Annual Review 1–10 https://www.anthonynolan.org/sites/default/files/2022-06/State%20of%20Th... (2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

