Dysregulated Wnt/β-catenin signaling confers resistance to cuproptosis in cancer cells
- PMID: 38987382
- PMCID: PMC11520902
- DOI: 10.1038/s41418-024-01341-2
Dysregulated Wnt/β-catenin signaling confers resistance to cuproptosis in cancer cells
Abstract
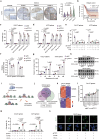
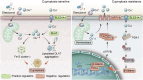
Cuproptosis is characterized by the aggregation of lipoylated enzymes of the tricarboxylic acid cycle and subsequent loss of iron-sulfur cluster proteins as a unique copper-dependent form of regulated cell death. As dysregulation of copper homeostasis can induce cuproptosis, there is emerging interest in exploiting cuproptosis for cancer therapy. However, the molecular drivers of cancer cell evasion of cuproptosis were previously undefined. Here, we found that cuproptosis activates the Wnt/β-catenin pathway. Mechanistically, copper binds PDK1 and promotes its interaction with AKT, resulting in activation of the Wnt/β-catenin pathway and cancer stem cell (CSC) properties. Notably, aberrant activation of Wnt/β-catenin signaling conferred resistance of CSCs to cuproptosis. Further studies showed the β-catenin/TCF4 transcriptional complex directly binds the ATP7B promoter, inducing its expression. ATP7B effluxes copper ions, reducing intracellular copper and inhibiting cuproptosis. Knockdown of TCF4 or pharmacological Wnt/β-catenin blockade increased the sensitivity of CSCs to elesclomol-Cu-induced cuproptosis. These findings reveal a link between copper homeostasis regulated by the Wnt/β-catenin pathway and cuproptosis sensitivity, and suggest a precision medicine strategy for cancer treatment through selective cuproptosis induction.
© 2024. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- 82072996/National Natural Science Foundation of China (National Science Foundation of China)
- 82273202/National Natural Science Foundation of China (National Science Foundation of China)
- 82103670/National Natural Science Foundation of China (National Science Foundation of China)
- 82002893/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

