Iron-sulphur protein catalysed [4+2] cycloadditions in natural product biosynthesis
- PMID: 38987535
- PMCID: PMC11236979
- DOI: 10.1038/s41467-024-50142-1
Iron-sulphur protein catalysed [4+2] cycloadditions in natural product biosynthesis
Abstract
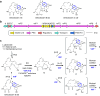
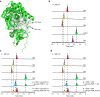
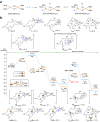
To the best of our knowledge, enzymes that catalyse intramolecular Diels-Alder ([4+2] cycloaddition) reactions are frequently reported in natural product biosynthesis; however, no native enzymes utilising Lewis acid catalysis have been reported. Verticilactam is a representative member of polycyclic macrolactams, presumably produced by spontaneous cycloaddition. We report that the intramolecular [4+2] cycloadditions can be significantly accelerated by ferredoxins (Fds), a class of small iron-sulphur (Fe-S) proteins. Through iron atom substitution by Lewis acidic gallium (Ga) iron and computational calculations, we confirm that the ubiquitous Fe-S cluster efficiently functions as Lewis acid to accelerate the tandem [4+2] cycloaddition and Michael addition reactions by lowering free energy barriers. Our work highlights Nature's ingenious strategy to generate complex molecule structures using the ubiquitous Fe-S protein. Furthermore, our study sheds light on the future design of Fd as a versatile Lewis acid catalyst for [4+2] cycloaddition reactions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tandem intermolecular [4 + 2] cycloadditions are catalysed by glycosylated enzymes for natural product biosynthesis.Nat Chem. 2023 Aug;15(8):1083-1090. doi: 10.1038/s41557-023-01260-8. Epub 2023 Jun 26. Nat Chem. 2023. PMID: 37365335
-
Enzyme-catalysed [6+4] cycloadditions in the biosynthesis of natural products.Nature. 2019 Apr;568(7750):122-126. doi: 10.1038/s41586-019-1021-x. Epub 2019 Mar 13. Nature. 2019. PMID: 30867595 Free PMC article.
-
Mechanistic insights into Diels-Alder reactions in natural product biosynthesis.Curr Opin Chem Biol. 2016 Dec;35:117-123. doi: 10.1016/j.cbpa.2016.09.015. Epub 2016 Sep 30. Curr Opin Chem Biol. 2016. PMID: 27697700 Review.
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
-
SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis.Nature. 2017 Sep 28;549(7673):502-506. doi: 10.1038/nature23882. Epub 2017 Sep 13. Nature. 2017. PMID: 28902839 Free PMC article.
Cited by
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
References
-
- Diels O, Alder K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928;460:98–122. doi: 10.1002/jlac.19284600106. - DOI
-
- Otto S, Engberts JBFN, Kwak JCT. Million-fold acceleration of a Diels–Alder reaction due to combined Lewis Acid and micellar catalysis in water. J. Am. Chem. Soc. 1998;120:9517–9525. doi: 10.1021/ja9816537. - DOI
-
- Woodward RB, Hoffmann R. The conservation of orbital symmetry. Angew. Chem. Int. Ed. 1969;8:781–853. doi: 10.1002/anie.196907811. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

