HLTF disrupts Cas9-DNA post-cleavage complexes to allow DNA break processing
- PMID: 38987539
- PMCID: PMC11237066
- DOI: 10.1038/s41467-024-50080-y
HLTF disrupts Cas9-DNA post-cleavage complexes to allow DNA break processing
Abstract
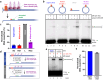
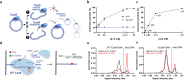
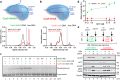
The outcome of CRISPR-Cas-mediated genome modifications is dependent on DNA double-strand break (DSB) processing and repair pathway choice. Homology-directed repair (HDR) of protein-blocked DSBs requires DNA end resection that is initiated by the endonuclease activity of the MRE11 complex. Using reconstituted reactions, we show that Cas9 breaks are unexpectedly not directly resectable by the MRE11 complex. In contrast, breaks catalyzed by Cas12a are readily processed. Cas9, unlike Cas12a, bridges the broken ends, preventing DSB detection and processing by MRE11. We demonstrate that Cas9 must be dislocated after DNA cleavage to allow DNA end resection and repair. Using single molecule and bulk biochemical assays, we next find that the HLTF translocase directly removes Cas9 from broken ends, which allows DSB processing by DNA end resection or non-homologous end-joining machineries. Mechanistically, the activity of HLTF requires its HIRAN domain and the release of the 3'-end generated by the cleavage of the non-target DNA strand by the Cas9 RuvC domain. Consequently, HLTF removes the H840A but not the D10A Cas9 nickase. The removal of Cas9 H840A by HLTF explains the different cellular impact of the two Cas9 nickase variants in human cells, with potential implications for gene editing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- ANR 23-CE11-0033/Agence Nationale de la Recherche (French National Research Agency)
- ANR-10-INBS-0005/Agence Nationale de la Recherche (French National Research Agency)
- 310030_207588/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- R35 GM122569/GM/NIGMS NIH HHS/United States
- R35 GM 122569/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
LinkOut - more resources
Full Text Sources

