Lipid-mediated intracellular delivery of recombinant bioPROTACs for the rapid degradation of undruggable proteins
- PMID: 38987546
- PMCID: PMC11237011
- DOI: 10.1038/s41467-024-50235-x
Lipid-mediated intracellular delivery of recombinant bioPROTACs for the rapid degradation of undruggable proteins
Abstract
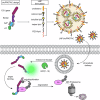
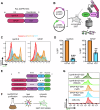
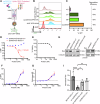
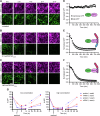
Recently, targeted degradation has emerged as a powerful therapeutic modality. Relying on "event-driven" pharmacology, proteolysis targeting chimeras (PROTACs) can degrade targets and are superior to conventional inhibitors against undruggable proteins. Unfortunately, PROTAC discovery is limited by warhead scarcity and laborious optimization campaigns. To address these shortcomings, analogous protein-based heterobifunctional degraders, known as bioPROTACs, have been developed. Compared to small-molecule PROTACs, bioPROTACs have higher success rates and are subject to fewer design constraints. However, the membrane impermeability of proteins severely restricts bioPROTAC deployment as a generalized therapeutic modality. Here, we present an engineered bioPROTAC template able to complex with cationic and ionizable lipids via electrostatic interactions for cytosolic delivery. When delivered by biocompatible lipid nanoparticles, these modified bioPROTACs can rapidly degrade intracellular proteins, exhibiting near-complete elimination (up to 95% clearance) of targets within hours of treatment. Our bioPROTAC format can degrade proteins localized to various subcellular compartments including the mitochondria, nucleus, cytosol, and membrane. Moreover, substrate specificity can be easily reprogrammed, allowing modular design and targeting of clinically-relevant proteins such as Ras, Jnk, and Erk. In summary, this work introduces an inexpensive, flexible, and scalable platform for efficient intracellular degradation of proteins that may elude chemical inhibition.
© 2024. The Author(s).
Conflict of interest statement
The University of Pennsylvania has filed a provisional patent application US 63/559,150 entitled “Lipid-Mediated Intracellular Delivery of Recombinant bioPROTACs for Rapid Degradation of Undruggable Proteins”, with Dr. Andrew Tsourkas and Alex Chan listed as inventors. The provisional patent covers the composition of the bioPROTAC and LNP described in this manuscript and uses thereof. The University of Pennsylvania has also filed patent application W02021077066A1 entitled “Lipid and lipid nanoparticle formulation for drug delivery”, which was published on 04-22-2021, with Dr. Michael Mitchell listed as an inventor. This patent describes several of the lipids and lipid nanoparticle compositions utilized in this manuscript, namely B6, C1, C14-2, C14-4, and C14-7. The remaining authors declare no competing interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

