Immune dysfunction prior to and during vaccination in multiple myeloma: a case study based on COVID-19
- PMID: 38987557
- PMCID: PMC11237013
- DOI: 10.1038/s41408-024-01089-5
Immune dysfunction prior to and during vaccination in multiple myeloma: a case study based on COVID-19
Abstract
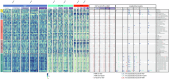
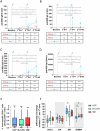
Infection is the leading cause of death in multiple myeloma (MM). However, the cellular composition associated with immune dysfunction is not defined. We analyzed immune profiles in the peripheral blood of patients with MM (n = 28) and B-cell chronic lymphoproliferative disorders (n = 53) vs. health care practitioners (n = 96), using multidimensional and computational flow cytometry. MM patients displayed altered distribution of most cell types (41/56, 73%), particularly within the B-cell (17/17) and T-cell (20/30) compartments. Using COVID-19 as a case study, we compared the immune response to vaccination based on 64,304 data points generated from the analysis of 1099 longitudinal samples. MM patients showed limited B-cell expansion linked to lower anti-RBD and anti-S antibody titers after the first two doses and booster. The percentages of B cells and CD4+ T cells in the blood, as well as the absolute counts of B cells and dendritic cells, predicted vaccine immunogenicity at different time points. In contrast with the humoral response, the percentage and antigen-dependent differentiation of SARS-CoV-2-specific CD8+ T cells was not altered in MM patients. Taken together, this study defined the cellular composition associated with immune dysfunction in MM and provided biomarkers such as the B-cell percentage and absolute count to individualize vaccination calendars.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lancman G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al. IVIg use associated with ten-fold reduction of serious infections in multiple myeloma patients treated with anti-BCMA bispecific antibodies. Blood Cancer Discov. 2023;4:440–51. doi: 10.1158/2643-3230.BCD-23-0049. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

