Caspase-8 promotes scramblase-mediated phosphatidylserine exposure and fusion of osteoclast precursors
- PMID: 38987568
- PMCID: PMC11237014
- DOI: 10.1038/s41413-024-00338-4
Caspase-8 promotes scramblase-mediated phosphatidylserine exposure and fusion of osteoclast precursors
Abstract
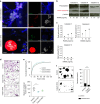
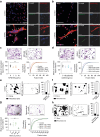
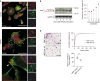
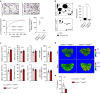
Efficient cellular fusion of mononuclear precursors is the prerequisite for the generation of fully functional multinucleated bone-resorbing osteoclasts. However, the exact molecular factors and mechanisms controlling osteoclast fusion remain incompletely understood. Here we identify RANKL-mediated activation of caspase-8 as early key event during osteoclast fusion. Single cell RNA sequencing-based analyses suggested that activation of parts of the apoptotic machinery accompanied the differentiation of osteoclast precursors into mature multinucleated osteoclasts. A subsequent characterization of osteoclast precursors confirmed that RANKL-mediated activation of caspase-8 promoted the non-apoptotic cleavage and activation of downstream effector caspases that translocated to the plasma membrane where they triggered activation of the phospholipid scramblase Xkr8. Xkr8-mediated exposure of phosphatidylserine, in turn, aided cellular fusion of osteoclast precursors and thereby allowed generation of functional multinucleated osteoclast syncytia and initiation of bone resorption. Pharmacological blockage or genetic deletion of caspase-8 accordingly interfered with fusion of osteoclasts and bone resorption resulting in increased bone mass in mice carrying a conditional deletion of caspase-8 in mononuclear osteoclast precursors. These data identify a novel pathway controlling osteoclast biology and bone turnover with the potential to serve as target for therapeutic intervention during diseases characterized by pathologic osteoclast-mediated bone loss. Proposed model of osteoclast fusion regulated by caspase-8 activation and PS exposure. RANK/RANK-L interaction. Activation of procaspase-8 into caspase-8. Caspase-8 activates caspase-3. Active capase-3 cleaves Xkr8. Local PS exposure is induced. Exposed PS is recognized by the fusion partner. FUSION. PS is re-internalized.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med.13, 791–801 (2007). - PubMed
-
- Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature397, 315–323 (1999). - PubMed
-
- Wada, T., Nakashima, T., Hiroshi, N. & Penninger, J. M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med.12, 17–25 (2006). - PubMed
-
- Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell3, 889–901 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

