A maternal brain hormone that builds bone
- PMID: 38987585
- PMCID: PMC11306098
- DOI: 10.1038/s41586-024-07634-3
A maternal brain hormone that builds bone
Abstract
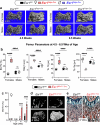
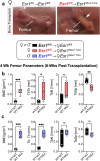
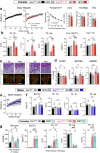
In lactating mothers, the high calcium (Ca2+) demand for milk production triggers significant bone loss1. Although oestrogen normally counteracts excessive bone resorption by promoting bone formation, this sex steroid drops precipitously during this postpartum period. Here we report that brain-derived cellular communication network factor 3 (CCN3) secreted from KISS1 neurons of the arcuate nucleus (ARCKISS1) fills this void and functions as a potent osteoanabolic factor to build bone in lactating females. We began by showing that our previously reported female-specific, dense bone phenotype2 originates from a humoral factor that promotes bone mass and acts on skeletal stem cells to increase their frequency and osteochondrogenic potential. This circulatory factor was then identified as CCN3, a brain-derived hormone from ARCKISS1 neurons that is able to stimulate mouse and human skeletal stem cell activity, increase bone remodelling and accelerate fracture repair in young and old mice of both sexes. The role of CCN3 in normal female physiology was revealed after detecting a burst of CCN3 expression in ARCKISS1 neurons coincident with lactation. After reducing CCN3 in ARCKISS1 neurons, lactating mothers lost bone and failed to sustain their progeny when challenged with a low-calcium diet. Our findings establish CCN3 as a potentially new therapeutic osteoanabolic hormone for both sexes and define a new maternal brain hormone for ensuring species survival in mammals.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
MeSH terms
Substances
Grants and funding
- R01 DK121657/DK/NIDDK NIH HHS/United States
- K01 AG065916/AG/NIA NIH HHS/United States
- K08 DK133674/DK/NIDDK NIH HHS/United States
- K12 GM081266/GM/NIGMS NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- R01 DK132073/DK/NIDDK NIH HHS/United States
- R01 AG070647/AG/NIA NIH HHS/United States
- K99 AG066963/AG/NIA NIH HHS/United States
- R00 AG066963/AG/NIA NIH HHS/United States
- R01 AG067740/AG/NIA NIH HHS/United States
- P30 AR075055/AR/NIAMS NIH HHS/United States
- R01 AG062331/AG/NIA NIH HHS/United States
- R56 AR081251/AR/NIAMS NIH HHS/United States
- R01 DK063592/DK/NIDDK NIH HHS/United States
- K99 DK129763/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

