An enterococcal phage-derived enzyme suppresses graft-versus-host disease
- PMID: 38987594
- PMCID: PMC11291292
- DOI: 10.1038/s41586-024-07667-8
An enterococcal phage-derived enzyme suppresses graft-versus-host disease
Abstract
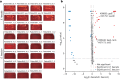
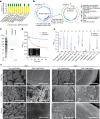
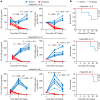
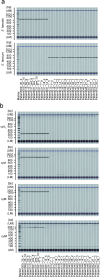
Changes in the gut microbiome have pivotal roles in the pathogenesis of acute graft-versus-host disease (aGVHD) after allogenic haematopoietic cell transplantation (allo-HCT)1-6. However, effective methods for safely resolving gut dysbiosis have not yet been established. An expansion of the pathogen Enterococcus faecalis in the intestine, associated with dysbiosis, has been shown to be a risk factor for aGVHD7-10. Here we analyse the intestinal microbiome of patients with allo-HCT, and find that E. faecalis escapes elimination and proliferates in the intestine by forming biofilms, rather than by acquiring drug-resistance genes. We isolated cytolysin-positive highly pathogenic E. faecalis from faecal samples and identified an anti-E. faecalis enzyme derived from E. faecalis-specific bacteriophages by analysing bacterial whole-genome sequencing data. The antibacterial enzyme had lytic activity against the biofilm of E. faecalis in vitro and in vivo. Furthermore, in aGVHD-induced gnotobiotic mice that were colonized with E. faecalis or with patient faecal samples characterized by the domination of Enterococcus, levels of intestinal cytolysin-positive E. faecalis were decreased and survival was significantly increased in the group that was treated with the E. faecalis-specific enzyme, compared with controls. Thus, administration of a phage-derived antibacterial enzyme that is specific to biofilm-forming pathogenic E. faecalis-which is difficult to eliminate with existing antibiotics-might provide an approach to protect against aGVHD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


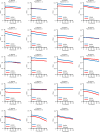

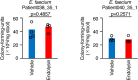
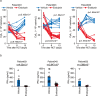
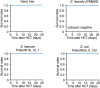
References
-
- Jones, J. M., Wilson, R. & Bealmear, P. M. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat. Res.45, 577–588 (1971). - PubMed
-
- van Bekkum, D. W., Roodenburg, J., Heidt, P. J. & van der Waaij, D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J. Natl Cancer Inst.52, 401–404 (1974). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

