In situ targeted base editing of bacteria in the mouse gut
- PMID: 38987595
- PMCID: PMC11338833
- DOI: 10.1038/s41586-024-07681-w
In situ targeted base editing of bacteria in the mouse gut
Abstract
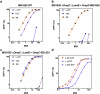
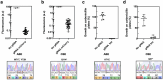
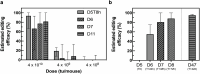
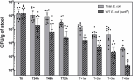
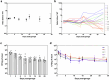
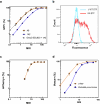
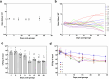
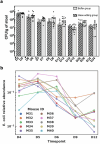
Microbiome research is now demonstrating a growing number of bacterial strains and genes that affect our health1. Although CRISPR-derived tools have shown great success in editing disease-driving genes in human cells2, we currently lack the tools to achieve comparable success for bacterial targets in situ. Here we engineer a phage-derived particle to deliver a base editor and modify Escherichia coli colonizing the mouse gut. Editing of a β-lactamase gene in a model E. coli strain resulted in a median editing efficiency of 93% of the target bacterial population with a single dose. Edited bacteria were stably maintained in the mouse gut for at least 42 days following treatment. This was achieved using a non-replicative DNA vector, preventing maintenance and dissemination of the payload. We then leveraged this approach to edit several genes of therapeutic relevance in E. coli and Klebsiella pneumoniae strains in vitro and demonstrate in situ editing of a gene involved in the production of curli in a pathogenic E. coli strain. Our work demonstrates the feasibility of modifying bacteria directly in the gut, offering a new avenue to investigate the function of bacterial genes and opening the door to the design of new microbiome-targeted therapies.
© 2024. The Author(s).
Conflict of interest statement
All authors are current or former employees, or paid advisors, of Eligo Bioscience. Eligo Bioscience owns US patent nos. 11,224,621, 11,376,286, 11,534,467 and 11,690,880 and international patent application no. WO2021/204967 relating to certain research described in this article.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

