Advances in virus-like particle-based SARS-CoV-2 vaccines
- PMID: 38988812
- PMCID: PMC11233461
- DOI: 10.3389/fcimb.2024.1406091
Advances in virus-like particle-based SARS-CoV-2 vaccines
Abstract
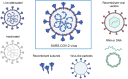
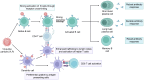
The Coronavirus Disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has incurred devastating human and economic losses. Vaccination remains the most effective approach for controlling the COVID-19 pandemic. Nonetheless, the sustained evolution of SARS-CoV-2 variants has provoked concerns among the scientific community regarding the development of next-generation COVID-19 vaccines. Among these, given their safety, immunogenicity, and flexibility to display varied and native epitopes, virus-like particle (VLP)-based vaccines represent one of the most promising next-generation vaccines. In this review, we summarize the advantages and characteristics of VLP platforms, strategies for antigen display, and current clinical trial progress of SARS-CoV-2 vaccines based on VLP platforms. Importantly, the experience and lessons learned from the development of SARS-CoV-2 VLP vaccines provide insights into the development of strategies based on VLP vaccines to prevent future coronavirus pandemics and other epidemics.
Keywords: COVID-19; SARS-CoV-2; immune response; receptor binding domain; spike protein; vaccines; virus-like particles.
Copyright © 2024 Hao, Yuan and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

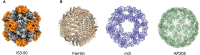
Similar articles
-
Dimming the corona: studying SARS-coronavirus-2 at reduced biocontainment level using replicons and virus-like particles.mBio. 2024 Dec 11;15(12):e0336823. doi: 10.1128/mbio.03368-23. Epub 2024 Nov 12. mBio. 2024. PMID: 39530689 Free PMC article. Review.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
Nanoparticle and virus-like particle vaccine approaches against SARS-CoV-2.J Microbiol. 2022 Mar;60(3):335-346. doi: 10.1007/s12275-022-1608-z. Epub 2022 Jan 28. J Microbiol. 2022. PMID: 35089583 Free PMC article. Review.
-
Safety, immunogenicity, and protection provided by unadjuvanted and adjuvanted formulations of a recombinant plant-derived virus-like particle vaccine candidate for COVID-19 in nonhuman primates.Cell Mol Immunol. 2022 Feb;19(2):222-233. doi: 10.1038/s41423-021-00809-2. Epub 2022 Jan 5. Cell Mol Immunol. 2022. PMID: 34983950 Free PMC article.
-
Development, production and characterization of SARS-CoV-2 virus-like particles (Coronaviridae: Orthocoronavirinae: Betacoronavirus: Sarbecovirus).Vopr Virusol. 2024 May 6;69(2):175-186. doi: 10.36233/0507-4088-226. Vopr Virusol. 2024. PMID: 38843023
Cited by
-
Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19.Int J Mol Sci. 2025 Jul 4;26(13):6462. doi: 10.3390/ijms26136462. Int J Mol Sci. 2025. PMID: 40650237 Free PMC article.
-
Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines.Vaccines (Basel). 2025 Feb 15;13(2):191. doi: 10.3390/vaccines13020191. Vaccines (Basel). 2025. PMID: 40006737 Free PMC article. Review.
-
Escherichia coli Nissle 1917 efficiently expresses the RBD domain of SARS-CoV-2 spike protein without codon optimization.Sci Rep. 2025 May 5;15(1):15670. doi: 10.1038/s41598-025-99902-z. Sci Rep. 2025. PMID: 40325187 Free PMC article.
-
Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern.Vaccines (Basel). 2025 Apr 17;13(4):424. doi: 10.3390/vaccines13040424. Vaccines (Basel). 2025. PMID: 40333293 Free PMC article. Review.
-
Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies.Vaccines (Basel). 2024 Aug 19;12(8):927. doi: 10.3390/vaccines12080927. Vaccines (Basel). 2024. PMID: 39204050 Free PMC article.
References
-
- Breitburd F., Kirnbauer R., Hubbert N. L., Nonnenmacher B., Trin-Dinh-Desmarquet C., Orth G., et al. (1995). Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69, 3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

