Contrast enhanced photoacoustic detection of fibrillar collagen in the near infrared region-I
- PMID: 38989511
- PMCID: PMC11232541
- DOI: 10.1039/d4na00204k
Contrast enhanced photoacoustic detection of fibrillar collagen in the near infrared region-I
Abstract
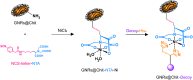
Fibrillar collagen accumulation emerges as a promising biomarker in several diseases, such as desmoplastic tumors and unstable atherosclerotic plaque. Gold nanorods (GNRs) hold great potential as contrast agents in high-resolution, biomedically safe, and non-invasive photoacoustic imaging (PAI). This study presents the design and characterization of a specialized imaging tool which exploits GNR assisted targeted photoacoustic imaging that is tailored for the identification of fibrillar collagen. In addition to the photoacoustic characterization of collagen in the NIR 1 and 2 regions, we demonstrate the detailed steps of conjugating a decoy to GNRs. This study serves as a proof of concept, that demonstrates that conjugated collagenase-1 (MMP-1) generates a distinct and collagen-specific photoacoustic signal, facilitating real-time visualization in the wavelength range of 700-970 nm (NIR I). As most of the reported studies utilized the endogenous contrast of collagen in the NIR II wavelength that has major limitations to perform in vivo deep tissue imaging, the approach that we are proposing is unique and it highlights the promise of MMP-1 decoy-functionalized GNRs as novel contrast agents for photoacoustic imaging of collagen in the NIR 1 region. To our knowledge this is the first time functionalized GNRs are optimized for the detection of fibrillar collagen and utilized in the field of non-invasive photoacoustic imaging that can facilitate a better prognosis of desmoplastic tumors and broken atherosclerotic plaques.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests; GM and JJ are employees of FujiFilm VisualSonics.
Figures





References
-
- Iacobuzio-Donahue C. A. Argani P. Hempen P. M. Jones J. Kern S. E. Cancer Res. 2002;62:5351–5357. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

