Amniotic Membrane-Derived Multichannel Hydrogels for Neural Tissue Repair
- PMID: 38989725
- PMCID: PMC12344635
- DOI: 10.1002/adhm.202400522
Amniotic Membrane-Derived Multichannel Hydrogels for Neural Tissue Repair
Abstract
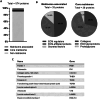
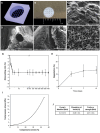
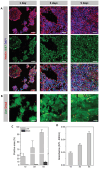
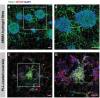
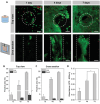
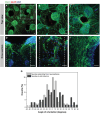
In the pursuit of advancing neural tissue regeneration, biomaterial scaffolds have emerged as promising candidates, offering potential solutions for nerve disruptions. Among these scaffolds, multichannel hydrogels, characterized by meticulously designed micrometer-scale channels, stand out as instrumental tools for guiding axonal growth and facilitating cellular interactions. This study explores the innovative application of human amniotic membranes modified with methacryloyl domains (AMMA) in neural stem cell (NSC) culture. AMMA hydrogels, possessing a tailored softness resembling the physiological environment, are prepared in the format of multichannel scaffolds to simulate native-like microarchitecture of nerve tracts. Preliminary experiments on AMMA hydrogel films showcase their potential for neural applications, demonstrating robust adhesion, proliferation, and differentiation of NSCs without the need for additional coatings. Transitioning into the 3D realm, the multichannel architecture fosters intricate neuronal networks guiding neurite extension longitudinally. Furthermore, the presence of synaptic vesicles within the cellular arrays suggests the establishment of functional synaptic connections, underscoring the physiological relevance of the developed neuronal networks. This work contributes to the ongoing efforts to find ethical, clinically translatable, and functionally relevant approaches for regenerative neuroscience.
Keywords: amniotic membrane; hydrogel; multichannel; nervous system; neural stem cells; scaffolds; tissue regeneration.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cohen‐Adad J., NeuroImage 2018, 182, 169. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

