Allergen-specific B cell responses in oral immunotherapy-induced desensitization, remission, and natural outgrowth in cow's milk allergy
- PMID: 38989779
- PMCID: PMC11724240
- DOI: 10.1111/all.16220
Allergen-specific B cell responses in oral immunotherapy-induced desensitization, remission, and natural outgrowth in cow's milk allergy
Abstract
Background: Antigen-specific memory B cells play a key role in the induction of desensitization and remission to food allergens in oral immunotherapy and in the development of natural tolerance (NT). Here, we characterized milk allergen Bos d 9-specific B cells in oral allergen-specific immunotherapy (OIT) and in children spontaneously outgrowing cow's milk allergy (CMA) due to NT.
Methods: Samples from children with CMA who received oral OIT (before, during, and after), children who naturally outgrew CMA (NT), and healthy individuals were received from Stanford biobank. Bos d 9-specific B cells were isolated by flow cytometry and RNA-sequencing was performed. Protein profile of Bos d 9-specific B cells was analyzed by proximity extension assay.
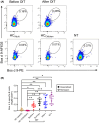
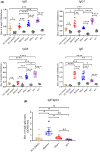
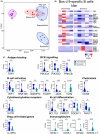
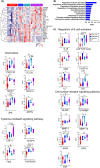
Results: Increased frequencies of circulating milk allergen Bos d 9-specific B cells were observed after OIT and NT. Milk-desensitized subjects showed the partial acquisition of phenotypic features of remission, suggesting that desensitization is an earlier stage of remission. Within these most significantly expressed genes, IL10RA and TGFB3 were highly expressed in desensitized OIT patients. In both the remission and desensitized groups, B cell activation-, Breg cells-, BCR-signaling-, and differentiation-related genes were upregulated. In NT, pathways associated with innate immunity characteristics, development of marginal zone B cells, and a more established suppressor function of B cells prevail that may play a role in long-term tolerance. The analyses of immunoglobulin heavy chain genes in specific B cells demonstrated that IgG2 in desensitization, IgG1, IgA1, IgA2, IgG4, and IgD in remission, and IgD in NT were predominating. Secreted proteins from allergen-specific B cells revealed higher levels of regulatory cytokines, IL-10, and TGF-β after OIT and NT.
Conclusion: Allergen-specific B cells are essential elements in regulating food allergy towards remission in OIT-received and naturally resolved individuals.
Keywords: Bos d 9 (Bos taurus domestic 9 or αS1‐casein); RNA sequencing; allergen‐specific B cells; cow's milk allergy; natural tolerance; oral immunotherapy (OIT).
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
C.A.A reports research grants from Allergopharma, Idorsia, GlaxoSmithKline, Novartis Research Institutes, AstraZeneca, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, grant from European Commission Horizon 2020 Framework Programme, Cure, advisory role in Sanofi/Regeneron, and SciBase, Sweden. M.S. reports research grants from the Swiss National Science Foundation (No. 310030_189334/1), GSK and Novartis and a speaker's fee from AstraZeneca. S.D.B. has consulted for Regeneron, Sanofi, Genent and Novartis on topics unrelated to this study, owns stock in Ab Cellera Biologics, has received grant support from the U.S. National Institutes of Health and the Bill and Melinda Gates Foundation, and has an endowment from the Crown Family Foundation. M.A has received research grants from the Swiss National Science Foundation (310,030_201,053/1), European Union (EU CURE, EU Syn‐Air‐G), is the Co‐Chair for EAACI Scientific Program; is on the Advisory Boards of Stanford University Sean Parker Asthma Allergy Center (CA, USA), LEO Foundation Skin Immunology Research Center Copenhagen, Denmark. W.V. has consulted for Mabylon AG on topics unrelated to this study and has received grant support from the Promedica foundation, Novartis Freenovation and the EoE foundation.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

