Synthesis of Simplified 2-Desmethyl Sanctolide A Analogs
- PMID: 38989836
- PMCID: PMC11414416
- DOI: 10.1021/acs.joc.4c00158
Synthesis of Simplified 2-Desmethyl Sanctolide A Analogs
Abstract
A one-pot, sequential phosphate tether-mediated method for the synthesis of simplified 2-desmethyl sanctolide A analogs is reported. Western side-chain diversification was achieved using a pot-efficient, sequential cross metathesis (CM)/ring-closing metathesis (RCM)/H2/dephosphorylation procedure. Further diversification was achieved by Me3Al-mediated amide formation, Yamaguchi esterification, and RCM macrocyclization to access five C11/C12 Z-configured, 2-des-methyl sanctolide A analogs with improved stability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




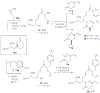


References
-
- Yadav JS; Suresh B; Srihari P Stereoselective total synthesis of the marine macrolide sanctolide A. Eur. J. Org. Chem 2015, 2015, 5856–5863.
-
- Wadsworth AD; Furkert DP; Brimble MA Total Synthesis of the Macrocyclic N-Methyl Enamides Palmyrolide A and 2 S-Sanctolide A. J. Org. Chem 2014, 79, 11179–11193. - PubMed
-
- Dissanayake GC; Ndi CN; Markley JL; Martinez J; Hanson PR Total Synthesis of Sanctolide A and Formal Synthesis of (2S)-Sanctolide A. J. Org. Chem 2023, 88, 805–817. - PMC - PubMed
- Dissanayake GC, Phosphate Tether-Mediated Strategies for the Synthesis of Complex Polyols, Natural Products, and Analogs. Ph.D. Thesis, University of Kansas, Lawrence, KS, 2022.
-
- Pereira AR; Cao Z; Engene N; Soria-Mercado IE; Murray TF; Gerwick WH Palmyrolide A, an Unusually Stabilized Neuroactive Macrolide from Palmyra Atoll Cyanobacteria. Org. Lett 2010, 12, 4490–4493 - PMC - PubMed
- Tello-Aburto R; Johnson EM; Valdez KC; Maio WA Asymmetric Total Synthesis and Absolute Stereochemistry of the Neuroactive Marine Macrolide Palmyrolide A. Org. Lett 2012, 14, 2150–2153.s - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

