Non-pharmaceutical interventions for COVID-19 transiently reduced pneumococcal and Haemophilus influenzae carriage in a cross-sectional pediatric cohort in Southampton, UK
- PMID: 38990033
- PMCID: PMC11302307
- DOI: 10.1128/spectrum.00224-24
Non-pharmaceutical interventions for COVID-19 transiently reduced pneumococcal and Haemophilus influenzae carriage in a cross-sectional pediatric cohort in Southampton, UK
Abstract
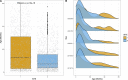
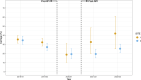
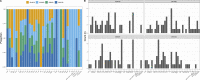
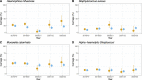
The Southampton pneumococcal carriage study of children under 5 years old continued during the coronavirus disease 2019 (COVID-19) pandemic. Here, we present data from October 2018 to March 2023 describing prevalence of pneumococci and other pathobionts during the winter seasons before, during, and after the introduction of non-pharmaceutical interventions (NPIs) to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission. Nasopharyngeal swabs were collected from children attending outpatient clinics at a secondary care hospital and community healthcare sites. Pre-NPIs, in 2019/2020, the carriage prevalence of pneumococci at the hospital site was 32% (n = 161 positive/499 participants). During NPIs, this fell to 19% (n = 12/64), although based on fewer participants compared to previous years due to COVID-19 restrictions on health-care attendance. In 2021/2022, after NPIs had eased, prevalence rebounded to 33% (n = 15/46) [compared to NPIs period, χ2 (1, N = 110) =2.78, P = 0.09]. Carriage prevalence at community healthcare sites fell significantly from 27% (n = 127/470) in 2019/2020 to 19% during the NPI period (n = 44/228) in 2020/2021 [χ2 (1, N = 698) =4.95, P = 0.026]. No rebound was observed in 2021/2022 [19% (n = 56/288)]. However, in a multivariate logistic regression model, neither site had a significantly lower carriage prevalence during the NPI period compared to the post NPI period. A reduction in serotype diversity was observed in 2020/2021. Carriage of Haemophilus influenzae was particularly affected by NPIs with a significant reduction observed. In conclusion, among children under 5 years of age, transient, modest, and statistically non-significant alterations in carriage of both Streptococcus pneumoniae and H. influenzae were associated with SARS-CoV-2 NPIs.IMPORTANCEStreptococcus pneumoniae (the pneumococcus) continues to be a major contributor to global morbidity and mortality. Using our long-running pediatric study, we examined changes in pneumococcal carriage prevalence in nearly 3,000 children under the age of 5 years between the winters of 2018/2019 and 2022/2023. This period coincided with the severe acute respiratory syndrome coronavirus 2 pandemic and, in particular, the implementation of national strategies to limit disease transmission in the UK. We observed a transient reduction of both Streptococcus pneumoniae and Haemophilus influenzae in these populations during this period of non-pharmaceutical interventions. This aligned with the reduction in invasive pneumococcal disease seen in the UK and is therefore a likely contributor to this phenomenon.
Keywords: COVID-19; Haemophilus influenzae; SARS-CoV2; Streptococcus pneumoniae; carriage.
Conflict of interest statement
D.W.C. was a post-doctoral researcher on GSK-funded projects in 2014/2915 and currently receives grant support from Pfizer. S.N.F. receives support from the National Institute for Health Research funding via the NIHR Southampton Clinical Research Facility and the NIHR Southampton Biomedical Research Centre. S.N.F. and S.C.C. act as principal investigators for clinical trials and other studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers. No personal payments are received from them. S.N.F. and S.C.C. have participated in advisory boards for vaccine manufacturers but receive no personal payments for this work. S.N.F. and S.C.C. have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS Trusts or Universities or to independent charities. J.C., M.L., K.H., J.S., and B.D.G. are employees of Pfizer and may hold stock and/or stock options.
Figures
References
-
- WHO director-general’s opening remarks at the media briefing on COVID-19. 2020. World Health Organization
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111. doi: 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- Brown J, Esme K-W, Carl B, Sarah B. 2021. Coronavirus: a history of English lockdown laws. House of Commons Library.
-
- Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD, Wenseleers T, Gimma A, Waites W, Wong KLM, van Zandvoort K, Silverman JD, Diaz-Ordaz K, Keogh R, Eggo RM, Funk S, Jit M, Atkins KE, Edmunds WJ, CMMID COVID-19 Working Group, COVID-19 Genomics UK (COG-UK) Consortium . 2021. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372:eabg3055. doi: 10.1126/science.abg3055 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

