Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways
- PMID: 38990057
- PMCID: PMC11238321
- DOI: 10.1111/jcmm.18386
Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways
Abstract
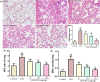
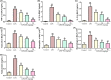
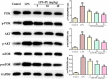
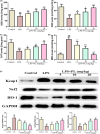
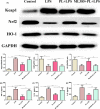
Acute lung injury (ALI) is a major pathophysiological problem characterized by severe inflammation, resulting in high morbidity and mortality. Plumbagin (PL), a major bioactive constituent extracted from the traditional Chinese herb Plumbago zeylanica, has been shown to possess anti-inflammatory and antioxidant pharmacological activities. However, its protective effect on ALI has not been extensively studied. The objective of this study was to investigate the protective effect of PL against ALI induced by LPS and to elucidate its possible mechanisms both in vivo and in vitro. PL treatment significantly inhibited pathological injury, MPO activity, and the wet/dry ratio in lung tissues, and decreased the levels of inflammatory cells and inflammatory cytokines TNF-α, IL-1β, IL-6 in BALF induced by LPS. In addition, PL inhibited the activation of the PI3K/AKT/mTOR signalling pathway, increased the activity of antioxidant enzymes CAT, SOD, GSH and activated the Keap1/Nrf2/HO-1 signalling pathway during ALI induced by LPS. To further assess the association between the inhibitory effects of PL on ALI and the PI3K/AKT/mTOR and Keap1/Nrf2/HO-1 signalling, we pretreated RAW264.7 cells with 740Y-P and ML385. The results showed that the activation of PI3K/AKT/mTOR signalling reversed the protective effect of PL on inflammatory response induced by LPS. Moreover, the inhibitory effects of PL on the production of inflammatory cytokines induced by LPS also inhibited by downregulating Keap1/Nrf2/HO-1 signalling. In conclusion, the results indicate that the PL ameliorate LPS-induced ALI by regulating the PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling, which may provide a novel therapeutic perspective for PL in inhibiting ALI.
Keywords: ALI; Keap1‐Nrf2/HO‐1 signalling; PI3K/AKT/mTOR signalling; plumbagin.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no financial interests to disclose.
Figures



Similar articles
-
Gen-17, a beta-methyl derivative of Genipin, attenuates LPS-induced ALI by regulating Keap1-Nrf2/HO-1 and suppressing NF-κB and MAPK-dependent signaling pathways.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167770. doi: 10.1016/j.bbadis.2025.167770. Epub 2025 Mar 3. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40037266
-
Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium.Int J Mol Sci. 2017 Feb 7;18(2):347. doi: 10.3390/ijms18020347. Int J Mol Sci. 2017. PMID: 28178212 Free PMC article.
-
Study on the mechanism of action of Penehyclidine hydrochloride on LPS-induced acute lung injury by regulating autophagy through the mTOR/Keap1/Nrf2 signaling pathway.J Pharm Biomed Anal. 2025 Sep 15;263:116938. doi: 10.1016/j.jpba.2025.116938. Epub 2025 Apr 27. J Pharm Biomed Anal. 2025. PMID: 40306137
-
Investigating the Interplay Between the Nrf2/Keap1/HO-1/SIRT-1 Pathway and the p75NTR/PI3K/Akt/MAPK Cascade in Neurological Disorders: Mechanistic Insights and Therapeutic Innovations.Mol Neurobiol. 2025 Jun;62(6):7597-7646. doi: 10.1007/s12035-025-04725-8. Epub 2025 Feb 7. Mol Neurobiol. 2025. PMID: 39920438 Review.
-
The Keap1/Nrf2/ARE/HO-1 axis in epilepsy: Crosstalk between oxidative stress and neuroinflammation.Int Immunopharmacol. 2025 Apr 24;153:114304. doi: 10.1016/j.intimp.2025.114304. Epub 2025 Mar 20. Int Immunopharmacol. 2025. PMID: 40117806 Review.
Cited by
-
Regulated programmed cell death in acute lung injury: from pathogenesis to therapy.Front Immunol. 2025 Jul 23;16:1630015. doi: 10.3389/fimmu.2025.1630015. eCollection 2025. Front Immunol. 2025. PMID: 40771815 Free PMC article. Review.
-
[Plumbagin protect against sepsis-induced myocardial injury in mice by inhibiting the JAK2/STAT3 signaling pathway to reduce cardiomyocyte pyroptosis].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Nov 20;44(11):2209-2219. doi: 10.12122/j.issn.1673-4254.2024.11.18. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39623277 Free PMC article. Chinese.
-
Natural Compounds Regulate Macrophage Polarization and Alleviate Inflammation Against ALI/ARDS.Biomolecules. 2025 Jan 29;15(2):192. doi: 10.3390/biom15020192. Biomolecules. 2025. PMID: 40001495 Free PMC article. Review.
-
Plumbagin ameliorates ferroptosis of ovarian granulosa cells in polycystic ovary syndrome by down-regulating SLC7A5 m6A methylation modification through inhibition of YTHDF1.J Ovarian Res. 2025 Jun 2;18(1):115. doi: 10.1186/s13048-025-01700-8. J Ovarian Res. 2025. PMID: 40457383 Free PMC article.
References
-
- Uemoto Y, Katsura T, Endo Y, et al. Genetic aspects of immunoglobulins and cyclophilin A in milk as potential indicators of mastitis resistance in Holstein cows. J Dairy Sci. 2023;107:1577‐1591. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

