Infection and the Glycome─New Insights into Host Response
- PMID: 38990078
- PMCID: PMC11320568
- DOI: 10.1021/acsinfecdis.4c00315
Infection and the Glycome─New Insights into Host Response
Abstract
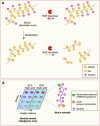
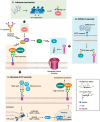
Glycans play critical roles in the host-pathogen interactions leading to infection. However, we still understand very little about the dynamic nature of glycosylation in response to infection and its function in modulating host immunity. Many of the host proteins involved in immune defense are glycoproteins. Furthermore, the innate immune system recognizes glycans. The glycoform of a protein can impact proteolytic stability, receptor interactions, serum half-life, and other aspects. New, cutting-edge chemical biology tools are shedding light on the interplay between infection and the host glycome. In this review, we highlight new work on the importance of dynamic glycosylation of host proteins in the innate and adaptive immune pathways in response to infection. These include recent findings on altered glycoprofiles of mucins, complement components, and antibodies.
Keywords: adaptive immunity; antibodies; carbohydrates; complement pathway; glycan; glycosylation; host response; innate immunity; macrophages; mucins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Brachyspira hyodysenteriae Infection Regulates Mucin Glycosylation Synthesis Inducing an Increased Expression of Core-2 O-Glycans in Porcine Colon.J Proteome Res. 2017 Apr 7;16(4):1728-1742. doi: 10.1021/acs.jproteome.7b00002. Epub 2017 Mar 30. J Proteome Res. 2017. PMID: 28301166
-
Aberrant Cellular Glycosylation May Increase the Ability of Influenza Viruses to Escape Host Immune Responses through Modification of the Viral Glycome.mBio. 2022 Apr 26;13(2):e0298321. doi: 10.1128/mbio.02983-21. Epub 2022 Mar 14. mBio. 2022. PMID: 35285699 Free PMC article.
-
Modulation of cellular tropism and innate antiviral response by viral glycans.J Innate Immun. 2009;1(5):405-12. doi: 10.1159/000226422. Epub 2009 Jun 25. J Innate Immun. 2009. PMID: 20375598 Free PMC article. Review.
-
Role of Protein Glycosylation in Host-Pathogen Interaction.Cells. 2020 Apr 20;9(4):1022. doi: 10.3390/cells9041022. Cells. 2020. PMID: 32326128 Free PMC article. Review.
-
The direct and indirect effects of glycans on immune function.Glycobiology. 2017 Jul 1;27(7):619-624. doi: 10.1093/glycob/cwx036. Glycobiology. 2017. PMID: 28460052 Review.
References
-
- Essentials of Glycobiology, 4th ed.; Varki A.; Cummings R. D.; Esko J. D.; Stanley P.; Hart G. W.; Aebi M.; Mohnen D.; Kinoshita T.; Packer N. H.; Prestegard J. H.; Schnaar R. L.; Seeberger P. H., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY; 2022. - PubMed
-
- Clausen T. M.; Sandoval D. R.; Spliid C. B.; Pihl J.; Perrett H. R.; Painter C. D.; Narayanan A.; Majowicz S. A.; Kwong E. M.; McVicar R. N.; Thacker B. E.; Glass C. A.; Yang Z.; Torres J. L.; Golden G. J.; Bartels P. L.; Porell R. N.; Garretson A. F.; Laubach L.; Feldman J.; Yin X.; Pu Y.; Hauser B. M.; Caradonna T. M.; Kellman B. P.; Martino C.; Gordts P. L. S. M.; Chanda S. K.; Schmidt A. G.; Godula K.; Leibel S. L.; Jose J.; Corbett K. D.; Ward A. B.; Carlin A. F.; Esko J. D. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183 (4), 1043–1057.e15. 10.1016/j.cell.2020.09.033. - DOI - PMC - PubMed
-
- Nguyen L.; McCord K. A.; Bui D. T.; Bouwman K. M.; Kitova E. N.; Elaish M.; Kumawat D.; Daskhan G. C.; Tomris I.; Han L.; Chopra P.; Yang T. J.; Willows S. D.; Mason A. L.; Mahal L. K.; Lowary T. L.; West L. J.; Hsu S. T. D.; Hobman T.; Tompkins S. M.; Boons G. J.; de Vries R. P.; Macauley M. S.; Klassen J. S. Sialic Acid-Containing Glycolipids Mediate Binding and Viral Entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18 (1), 81–90. 10.1038/s41589-021-00924-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

