The C-terminus of Rad is required for membrane localization and L-type calcium channel regulation
- PMID: 38990175
- PMCID: PMC11244639
- DOI: 10.1085/jgp.202313518
The C-terminus of Rad is required for membrane localization and L-type calcium channel regulation
Abstract
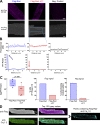
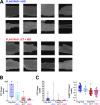
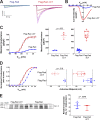
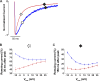
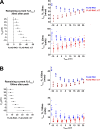
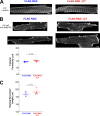
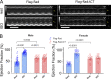
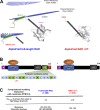
L-type CaV1.2 current (ICa,L) links electrical excitation to contraction in cardiac myocytes. ICa,L is tightly regulated to control cardiac output. Rad is a Ras-related, monomeric protein that binds to L-type calcium channel β subunits (CaVβ) to promote inhibition of ICa,L. In addition to CaVβ interaction conferred by the Rad core motif, the highly conserved Rad C-terminus can direct membrane association in vitro and inhibition of ICa,L in immortalized cell lines. In this work, we test the hypothesis that in cardiomyocytes the polybasic C-terminus of Rad confers t-tubular localization, and that membrane targeting is required for Rad-dependent ICa,L regulation. We introduced a 3xFlag epitope to the N-terminus of the endogenous mouse Rrad gene to facilitate analysis of subcellular localization. Full-length 3xFlag-Rad (Flag-Rad) mice were compared with a second transgenic mouse model, in which the extended polybasic C-termini of 3xFlag-Rad was truncated at alanine 277 (Flag-RadΔCT). Ventricular cardiomyocytes were isolated for anti-Flag-Rad immunocytochemistry and ex vivo electrophysiology. Full-length Flag-Rad showed a repeating t-tubular pattern whereas Flag-RadΔCT failed to display membrane association. ICa,L in Flag-RadΔCT cardiomyocytes showed a hyperpolarized activation midpoint and an increase in maximal conductance. Additionally, current decay was faster in Flag-RadΔCT cells. Myocardial ICa,L in a Rad C-terminal deletion model phenocopies ICa,L modulated in response to β-AR stimulation. Mechanistically, the polybasic Rad C-terminus confers CaV1.2 regulation via membrane association. Interfering with Rad membrane association constitutes a specific target for boosting heart function as a treatment for heart failure with reduced ejection fraction.
© 2024 Elmore et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures



References
-
- Béguin, P., Mahalakshmi R.N., Nagashima K., Cher D.H., Takahashi A., Yamada Y., Seino Y., and Hunziker W.. 2005. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J. Cell Sci. 118:1923–1934. 10.1242/jcs.02321 - DOI - PubMed
-
- Béguin, P., Mahalakshmi R.N., Nagashima K., Cher D.H., Ikeda H., Yamada Y., Seino Y., and Hunziker W.. 2006. Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J. Mol. Biol. 355:34–46. 10.1016/j.jmb.2005.10.013 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

