Momelotinib versus Continued Ruxolitinib or Best Available Therapy in JAK Inhibitor-Experienced Patients with Myelofibrosis and Anemia: Subgroup Analysis of SIMPLIFY-2
- PMID: 38990433
- PMCID: PMC11349857
- DOI: 10.1007/s12325-024-02928-4
Momelotinib versus Continued Ruxolitinib or Best Available Therapy in JAK Inhibitor-Experienced Patients with Myelofibrosis and Anemia: Subgroup Analysis of SIMPLIFY-2
Abstract
Introduction: Some Janus kinase (JAK) inhibitors such as ruxolitinib and fedratinib do not address and may worsen anemia in patients with myelofibrosis. In these cases, the JAK inhibitor may be continued at a reduced dose in an effort to maintain splenic and symptom control, with supportive therapy and/or red blood cell (RBC) transfusions added to manage anemia. This post hoc descriptive analysis of the phase 3 SIMPLIFY-2 trial evaluated the relative benefits of this approach versus switching to the JAK1/JAK2/activin A receptor type 1 inhibitor momelotinib in patients for whom anemia management is a key consideration.
Methods: SIMPLIFY-2 was a randomized (2:1), open-label, phase 3 trial of momelotinib versus best available therapy (BAT; 88.5% continued ruxolitinib) in JAK inhibitor-experienced patients with myelofibrosis (n = 156). Patient subgroups (n = 105 each) were defined by either baseline (1) hemoglobin (Hb) of < 100 g/L or (2) non-transfusion independence (not meeting the criteria of no transfusions and no Hb of < 80 g/L for the previous 12 weeks); outcomes have been summarized descriptively.
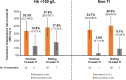
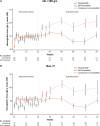
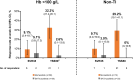
Results: In both subgroups of interest, week 24 transfusion independence rates were higher with momelotinib versus BAT/ruxolitinib: baseline Hb of < 100 g/L, 22 (33.3%) versus 5 (12.8%); baseline non-transfusion independent, 25 (34.7%) versus 1 (3.0%). Mean Hb levels over time were also generally higher in both subgroups with momelotinib, despite median transfusion rates through week 24 with momelotinib being comparable to or lower than with BAT/ruxolitinib. Spleen and symptom response rates with momelotinib in these subgroups were comparable to the intent-to-treat population, while rates with BAT/ruxolitinib were lower.
Conclusion: In patients with moderate-to-severe anemia and/or in need of RBC transfusions, outcomes were improved by switching to momelotinib rather than continuing ruxolitinib and using anemia supportive therapies.
Trial registration: ClinicalTrials.gov: NCT02101268.
Keywords: Anemia; Anemia supportive therapy; Erythropoiesis-stimulating agents; Hemoglobin; Momelotinib; Myelofibrosis; Red blood cell transfusions; Ruxolitinib; Transfusion independence.
Plain language summary
Patients with the rare blood cancer myelofibrosis often experience symptoms such as tiredness, an increase in the size of their spleens (an organ involved in filtering the blood), and anemia (too few red blood cells). One type of treatment for myelofibrosis, called a Janus kinase (JAK) inhibitor, can help patients to feel better and reduce the size of their spleens, but some JAK inhibitors do not help with anemia and may make it worse. In those situations, patients may continue to take their JAK inhibitor but also receive another type of treatment, called an anemia supportive therapy, and may also receive red blood cell transfusions. This study compared 2 treatment approaches, continuing the JAK inhibitor ruxolitinib and adding an anemia supportive therapy and/or transfusions versus switching to another treatment called momelotinib, in 2 groups of patients from a clinical trial: (1) patients with levels of hemoglobin (a red blood cell protein) at the start of the trial that indicated that they had anemia, and (2) patients who were already receiving red blood cell transfusions at the start of the trial. In both groups, more patients did not need red blood cell transfusions anymore at week 24 with momelotinib, and their hemoglobin levels on average became higher over time. More patients also had improvements in spleen size and symptoms with momelotinib. Overall, outcomes were improved by switching to momelotinib rather than continuing ruxolitinib and using supportive therapies and/or red blood cell transfusions to treat anemia.
© 2024. Crown.
Conflict of interest statement
Claire N. Harrison reports consultancy with Novartis, MorphoSys, Sierra, Constellation, AOP, Galecto, Keros, AbbVie, Geron, Janssen, Promedior, Sumitomo, GSK, and CTI; participation in a company-sponsored speakers’ bureau for AbbVie, BMS, Celgene, CTI, and Novartis; research funding from Constellation, Novartis, Celgene (BMS), and Imago Biosciences, Inc., a subsidiary of Merck & Co.; sole ownership and directorship of Chakana Medical Limited; advisory roles with Galecto and Keros; patents and royalties from Blood Cancer UK, BMS, Chakana Medical, Constellation, EHA, MPN Voice, and Novartis; and honoraria from BMS, Novartis, CTI, GSK, AOP, Galecto, AbbVie, and MorphoSys. Alessandro M. Vannucchi reports honoraria from Incyte, Novartis, BMS, GSK, AbbVie, Roche, and AOP. Christian Recher reports honoraria from and an advisory role with AbbVie, Astellas, Amgen, BMS, Jazz Pharmaceuticals, Novartis, and Servier and research funding from AbbVie, Astellas, Amgen, BMS, Jazz Pharmaceuticals, and Servier. Francesco Passamonti reports honoraria from AbbVie, AOP Orphan, and Bristol Myers Squibb/Celgene and consultancy with AbbVie, AOP Orphan, Celgene, Bristol Myers Squibb, Janssen, Kartos, Karyopharm, Kyowa Kirin, MEI, Novartis, Roche, Sierra Oncology, and Sumitomo. Aaron T. Gerds reports consultancy with AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals, GSK, Kartos, Novartis, PharmaEssentia, and Sierra Oncology; consultancy with and research funding from CTI BioPharma, Imago BioSciences/Merck, and Constellation Pharmaceuticals/MorphoSys; research funding from Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, and Kartos Pharmaceuticals; and an advisory role with AbbVie, Bristol Myers Squibb, CTI BioPharma, GSK, Imago, Kartos, MorphoSys, PharmaEssentia, and Rain Oncology. Juan Carlos Hernandez-Boluda reports an advisory role with Incyte, Pfizer, Novartis, and BMS. Abdulraheem Yacoub reports consultancy with Incyte, CTI Pharma, PharmaEssentia, Pfizer, Novartis, and Acceleron Pharma. Shireen Sirhan reports honoraria from Constellation and consultancy with and honoraria from GSK, Novartis, and Constellation. Catherine Ellis, Bharat Patel, and Bryan Strouse are employees of GSK. Uwe Platzbecker reports consultancy with AbbVie, Curis, and Geron and an advisory role with MDS Foundation. All authors acknowledge editorial support in the preparation of this manuscript, funded by GSK.
Figures
Similar articles
-
Longitudinal Assessment of Transfusion Intensity in Patients With JAK Inhibitor-Naive or -Experienced Myelofibrosis Treated With Momelotinib.Clin Lymphoma Myeloma Leuk. 2025 Mar;25(3):199-211. doi: 10.1016/j.clml.2024.10.001. Epub 2024 Oct 16. Clin Lymphoma Myeloma Leuk. 2025. PMID: 39516087 Clinical Trial.
-
Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial.Lancet Haematol. 2018 Feb;5(2):e73-e81. doi: 10.1016/S2352-3026(17)30237-5. Epub 2017 Dec 20. Lancet Haematol. 2018. PMID: 29275119 Clinical Trial.
-
Momelotinib vs. ruxolitinib in myelofibrosis patient subgroups by baseline hemoglobin levels in the SIMPLIFY-1 trial.Leuk Lymphoma. 2024 Jul;65(7):965-977. doi: 10.1080/10428194.2024.2328800. Epub 2024 Mar 19. Leuk Lymphoma. 2024. PMID: 38501751 Clinical Trial.
-
Momelotinib for the treatment of myelofibrosis with anemia.Future Oncol. 2022 Jun;18(20):2559-2571. doi: 10.2217/fon-2022-0276. Epub 2022 May 23. Future Oncol. 2022. PMID: 35603634 Review.
-
Momelotinib: an emerging treatment for myelofibrosis patients with anemia.J Hematol Oncol. 2022 Jan 19;15(1):7. doi: 10.1186/s13045-021-01157-4. J Hematol Oncol. 2022. PMID: 35045875 Free PMC article. Review.
Cited by
-
Transfusion-Related Cost and Time Burden Offsets in Patients with Myelofibrosis Treated with Momelotinib in the SIMPLIFY-1 and SIMPLIFY-2 Trials.Cancers (Basel). 2024 Dec 5;16(23):4067. doi: 10.3390/cancers16234067. Cancers (Basel). 2024. PMID: 39682253 Free PMC article.
-
When, which and how to switch: Navigating JAK inhibitors in myelofibrosis.Br J Haematol. 2024 Nov 27:10.1111/bjh.19929. doi: 10.1111/bjh.19929. Online ahead of print. Br J Haematol. 2024. PMID: 39604140 Free PMC article. Review.
-
Emerging Therapeutic Approaches for Anemia in Myelofibrosis.Curr Hematol Malig Rep. 2025 May 3;20(1):7. doi: 10.1007/s11899-025-00751-4. Curr Hematol Malig Rep. 2025. PMID: 40317385 Review.
-
Spatial-transcriptomic profiling: a new lens for understanding myelofibrosis pathophysiology.Cell Commun Signal. 2024 Oct 21;22(1):510. doi: 10.1186/s12964-024-01877-3. Cell Commun Signal. 2024. PMID: 39434124 Free PMC article. Review.
-
Anemia in Myelofibrosis: A Focus on Proactive Management and the Role of Momelotinib.Cancers (Basel). 2024 Dec 4;16(23):4064. doi: 10.3390/cancers16234064. Cancers (Basel). 2024. PMID: 39682250 Free PMC article. Review.
References
-
- Jakafi (ruxolitinib). Prescribing information. Incyte; 2023. https://www.jakafi.com/pdf/prescribing-information.pdf. Accessed Apr 22, 2024.
-
- Inrebic (fedratinib). Prescribing information. Bristol Myers Squibb; 2023. https://packageinserts.bms.com/pi/pi_inrebic.pdf. Accessed Apr 22, 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

