Anticancer therapeutic potential of multimodal targeting agent- "phosphorylated galactosylated chitosan coated magnetic nanoparticles" against N-nitrosodiethylamine-induced hepatocellular carcinoma
- PMID: 38990437
- PMCID: PMC11782354
- DOI: 10.1007/s13346-024-01655-1
Anticancer therapeutic potential of multimodal targeting agent- "phosphorylated galactosylated chitosan coated magnetic nanoparticles" against N-nitrosodiethylamine-induced hepatocellular carcinoma
Abstract
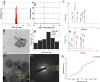
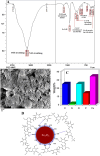
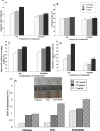
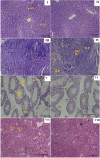
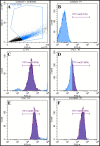
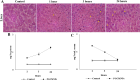
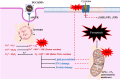
Superparamagnetic iron oxide nanoparticles (SPIONs) are extensively used as carriers in targeted drug delivery and has several advantages in the field of magnetic hyperthermia, chemodynamic therapy and magnet assisted radionuclide therapy. The characteristics of SPIONs can be tailored to deliver drugs into tumor via "passive targeting" and they can also be coated with tissue-specific agents to enhance tumor uptake via "active targeting". In our earlier studies, we developed HCC specific targeting agent- "phosphorylated galactosylated chitosan"(PGC) for targeting asialoglycoprotein receptors. Considering their encouraging results, in this study we developed a multifunctional targeting system- "phosphorylated galactosylated chitosan-coated magnetic nanoparticles"(PGCMNPs) for targeting HCC. PGCMNPs were synthesized by co-precipitation method and characterized by DLS, XRD, TEM, VSM, elemental analysis and FT-IR spectroscopy. PGCMNPs were evaluated for in vitro antioxidant properties, uptake in HepG2 cells, biodistribution, in vivo toxicity and were also evaluated for anticancer therapeutic potential against NDEA-induced HCC in mice model in terms of tumor status, electrical properties, antioxidant defense status and apoptosis. The characterization studies confirmed successful formation of PGCMNPs with superparamagnetic properties. The internalization studies demonstrated (99-100)% uptake of PGCMNPs in HepG2 cells. These results were also supported by biodistribution studies in which increased iron content (296%) was noted inside the hepatocytes. Further, PGCMNPs exhibited no in vivo toxicity. The anticancer therapeutic potential was evident from observation that PGCMNPs treatment decreased tumor bearing animals (41.6%) and significantly (p ≤ 0.05) lowered tumor multiplicity. Overall, this study indicated that PGCMNPs with improved properties are efficiently taken-up by hepatoma cells and has therapeutic potential against HCC. Further, this agent can be tagged with 32P and hence can offer multimodal cancer treatment options via radiation ablation as well as magnetic hyperthermia.
Keywords: Asialoglycoprotein receptor; Liver cancer; NDEA; Superparamagnetic iron oxide nanoparticles; Targeted drug delivery.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval statement: In order to study the in vivo toxicity and in vivo anticancer ability of PGCMNPs, the usage of animals were necessary. All animal experiments were performed as per the guidelines of Committee for the Control and Supervision of experiments on animals (IAEC/KMC/85/2017) (Manipal Academy of Higher Education, Manipal) and ARRIVE guidelines. Experiments were conducted under controlled conditions (temperature: 25±2°C, humidity: 65-80%) with 12 hours of light and dark cycle. Animals were provided with ad libitum supply of clean drinking water and standard animal pellet diet (VRK nutritional solutions, Maharashtra, India). After the completion of respective period of treatments animals were euthanized using large dose of thiopental sodium (50 mg/kg bw, intraperitoneal). Competing interests: The authors report that there are no competing interests to declare.
Figures

Similar articles
-
In vitro and in vivo evaluation of self-assembled chitosan nanoparticles selectively overcoming hepatocellular carcinoma via asialoglycoprotein receptor.Drug Deliv. 2021 Dec;28(1):2071-2084. doi: 10.1080/10717544.2021.1983077. Drug Deliv. 2021. PMID: 34595970 Free PMC article.
-
Lactosylated Chitosan Nanoparticles Potentiate the Anticancer Effects of Telmisartan In Vitro and in a N-Nitrosodiethylamine-Induced Mice Model of Hepatocellular Carcinoma.Mol Pharm. 2023 Sep 4;20(9):4758-4769. doi: 10.1021/acs.molpharmaceut.3c00542. Epub 2023 Aug 16. Mol Pharm. 2023. PMID: 37585079
-
Anticancer therapeutic potential of phosphorylated galactosylated chitosan against N-nitrosodiethyl amine-induced hepatocarcinogenesis.Arch Biochem Biophys. 2022 Oct 15;728:109375. doi: 10.1016/j.abb.2022.109375. Epub 2022 Aug 12. Arch Biochem Biophys. 2022. PMID: 35970414
-
Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma.World J Gastroenterol. 2015 Nov 14;21(42):12022-41. doi: 10.3748/wjg.v21.i42.12022. World J Gastroenterol. 2015. PMID: 26576089 Free PMC article. Review.
-
Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers.Int J Nanomedicine. 2012;7:3445-71. doi: 10.2147/IJN.S30320. Epub 2012 Jul 6. Int J Nanomedicine. 2012. PMID: 22848170 Free PMC article. Review.
References
-
- Kanwar N, Anushree U, Divya KP, Singh SP, Bharati S, Kanwal A. Biological systems and nanopharmacokinetics. In: Nano-pharmacokinetics and theranostics. Cambridge: Academic Press; 2021. pp. 153–170. 10.1016/B978-0-323-85050-6.00010-4.
-
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–98. - PubMed
-
- Zi, Y., Yang, K., He, J., Wu, Z., Liu, J., & Zhang, W. (2022). Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv Drug Delivery Rev., 114449. 10.1016/j.addr.2022.114449. - PubMed
-
- Cui W, Li J, Decher G. Self-assembled smart nanocarriers for targeted drug delivery. Adv Mater. 2016;28(6):1302–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

