Outcomes Among Racial and Ethnic Minority Patients With Advanced Cancers in Phase 1 Trials: A Meta-Analysis
- PMID: 38990570
- PMCID: PMC11240188
- DOI: 10.1001/jamanetworkopen.2024.21485
Outcomes Among Racial and Ethnic Minority Patients With Advanced Cancers in Phase 1 Trials: A Meta-Analysis
Erratum in
-
Error in Author Degree.JAMA Netw Open. 2024 Aug 1;7(8):e2433744. doi: 10.1001/jamanetworkopen.2024.33744. JAMA Netw Open. 2024. PMID: 39163052 Free PMC article. No abstract available.
Abstract
Importance: Patients from racial and ethnic minority groups (eg, Asian, Hispanic, and non-Hispanic Black patients) have low representation in clinical trials, especially in phase 1 trials in cancer. These trials represent valuable options for patients with advanced cancer who experience disease progression with standard therapy.
Objective: To determine whether the benefit of enrollment to phase 1 cancer trials extends to Asian, Hispanic, and non-Hispanic Black patients as much as it does for non-Hispanic White patients.
Data sources: Patient records at a single institution from January 1999 to December 2016 were reviewed. Treatment-related responses, toxic effects, and deaths were recorded.
Study selection: All phase 1 studies were included.
Data extraction and synthesis: Data underwent independent extraction by multiple observers following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main outcomes and measures: The primary outcome was overall survival (OS), assessed using univariate and multivariable time-to-event analyses.
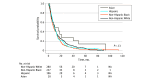
Results: A total of 738 patients (median [range], 60 [22-93] years; 467 [63.3] female) including 197 Hispanic patients (26.7%), 238 non-Hispanic Black patients (32.2%), and 282 non-Hispanic White patients (38.2%), were enrolled in 64 phase 1 trials, including 33 cytotoxic trials (51.5%), 21 biologic trials (32.8%), and 10 combined therapy trials (15.6%). The primary cancer diagnoses were colorectal (187 patients [25.3%]), ovarian (141 patients [19.1%]), lung (58 patients [7.9%]), uterine (49 patients [6.6%]), and breast (41 patients [5.6%]). Patients underwent a median (range) of 3 (0-13) therapies prior to trial enrollment. Among 558 patients evaluated for response, the clinical benefit rate (ie, stable disease plus response rates) was 49.1%, and the overall response rate was 6.5%. Grade 3 or 4 nonhematological toxic effects were observed in 27.8% (95% CI, 24.6%-31.3%) of patients and grade 3 or 4 hematological toxic effects were observed in 19.7% (95% CI, 17.0%-22.8%) of patients. The treatment-related mortality rate was 0.9% (95% CI, 0.4%-1.9%). Median OS was 9.6 (95% CI, 8.2-11.0) months among Hispanic patients, 8.3 (95% CI, 6.7-10.4) months among non-Hispanic Black patients, and 9.8 (95% CI, 8.5-11.4) months among non-Hispanic White patients (P = .13). In a multivariable analysis, age older than 60 years, Eastern Cooperative Oncology Group performance status score of 2 or greater, more than 2 metastatic sites, lactate dehydrogenase grade 1 or 2, grade 2 or greater low albumin, grade 1 or greater total bilirubin, and grade 2 or greater anemia were associated with worse prognosis, whereas leukocytosis greater than grade 1 was associated with better OS.
Conclusions and relevance: In this meta-analysis assessing outcomes in phase 1 cancer trials among patients from racial and ethnic minority groups, Hispanic and non-Hispanic Black patients had benefits similar to those of non-Hispanic White patients.
Conflict of interest statement
Figures
Similar articles
-
Disparities in the Inclusion of Racial and Ethnic Minority Groups and Older Adults in Prostate Cancer Clinical Trials: A Meta-analysis.JAMA Oncol. 2023 Feb 1;9(2):180-187. doi: 10.1001/jamaoncol.2022.5511. JAMA Oncol. 2023. PMID: 36416812 Free PMC article.
-
Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 May 3;4(5):e218348. doi: 10.1001/jamanetworkopen.2021.8348. JAMA Netw Open. 2021. PMID: 34003274 Free PMC article.
-
Quality of Life After Axillary Lymph Node Dissection Among Racial and Ethnic Minority Women.JAMA Surg. 2024 Jun 1;159(6):668-676. doi: 10.1001/jamasurg.2024.0118. JAMA Surg. 2024. PMID: 38536186 Free PMC article.
-
Differences in Stage of Cancer at Diagnosis, Treatment, and Survival by Race and Ethnicity Among Leading Cancer Types.JAMA Netw Open. 2020 Apr 1;3(4):e202950. doi: 10.1001/jamanetworkopen.2020.2950. JAMA Netw Open. 2020. PMID: 32267515 Free PMC article.
-
Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials.JAMA Netw Open. 2021 Feb 1;4(2):e2037640. doi: 10.1001/jamanetworkopen.2020.37640. JAMA Netw Open. 2021. PMID: 33606033 Free PMC article. Review.
Cited by
-
Error in Author Degree.JAMA Netw Open. 2024 Aug 1;7(8):e2433744. doi: 10.1001/jamanetworkopen.2024.33744. JAMA Netw Open. 2024. PMID: 39163052 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical