The antimicrobial activity of an antiseptic soap against Candida Albicans and Streptococcus Mutans single and dual-species biofilms on denture base and reline acrylic resins
- PMID: 38990802
- PMCID: PMC11239035
- DOI: 10.1371/journal.pone.0306862
The antimicrobial activity of an antiseptic soap against Candida Albicans and Streptococcus Mutans single and dual-species biofilms on denture base and reline acrylic resins
Erratum in
-
Correction: The antimicrobial activity of an antiseptic soap against Candida Albicans and Streptococcus Mutans single and dual-species biofilms on denture base and reline acrylic resins.PLoS One. 2025 Mar 13;20(3):e0320387. doi: 10.1371/journal.pone.0320387. eCollection 2025. PLoS One. 2025. PMID: 40080517 Free PMC article.
Abstract
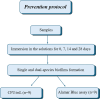
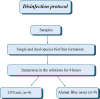
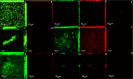
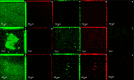
To evaluate the effect of antiseptic soap on single and dual-species biofilms of Candida albicans and Streptococcus mutans on denture base and reline resins. Samples of the resins were distributed into groups (n = 9) according to the prevention or disinfection protocols. In the prevention protocol, samples were immersed in the solutions (Lifebuoy, 0.5% sodium hypochlorite solution and PBS) for 7, 14 and 28 days before the single and dual-species biofilms formation. Overnight denture disinfection was simulated. In the disinfection protocol, samples were immersed in the same solutions during 8 hours after the single and dual-species biofilms formation. Antimicrobial activity was analyzed by counting colony-forming units (CFU/mL) and evaluating cell metabolism. Cell viability and protein components of the biofilm matrix were evaluated using confocal laser scanning microscopy (CLSM). Data were submitted to ANOVA, followed by Tukey's post-test (α = 0.05) or Dunnett's T3 multiple comparisons test. In the prevention protocol, Lifebuoy solution effectively reduced the number of CFU/mL of both species. In addition, the solution decreased the cell metabolism of the microorganisms. Regarding disinfection protocol, the Lifebuoy solution was able of reduce approximately of 2-3 logs for all the biofilms on the denture base and reline resin. Cellular metabolism was also reduced. The images obtained with CLSM corroborate these results. Lifebuoy solution was effective in reducing single and dual-species biofilms on denture base and reline resins.
Copyright: © 2024 Tasso et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

