A low molecular weight dextran sulphate, ILB®, for the treatment of amyotrophic lateral sclerosis (ALS): An open-label, single-arm, single-centre, phase II trial
- PMID: 38990927
- PMCID: PMC11239073
- DOI: 10.1371/journal.pone.0291285
A low molecular weight dextran sulphate, ILB®, for the treatment of amyotrophic lateral sclerosis (ALS): An open-label, single-arm, single-centre, phase II trial
Abstract
Background: Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig´s disease, is a rare neurological condition and is the most common motor neurone disease. It is a fatal disease with specific loss of motor neurons in the spinal cord, brain stem, and motor cortex leading to progressive paralysis and usually death within five years of diagnosis. There remains no cure for ALS, and management is focused on a combination of neuroprotective medication, respiratory support, and management by multidisciplinary clinics.
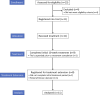
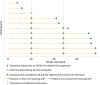
Patients and methods: This prospective, single-arm, open-label phase II clinical trial of sustained weekly administration of 2 mg/kg ILB® (a low-molecular weight dextran sulphate) was conducted in a single UK hospital. Eligible patients were at least 18 years and had a definite diagnosis of ALS according to El Escorial Criteria. The co-primary outcomes were safety, tolerability, and quantity of ILB® administered. EudraCT number. 2018-000668-28.
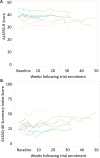
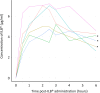
Findings: Between 18-Apr-2019 and 27-Mar-2020, 11 patients were recruited and treated for up to 38 weeks. There were no treatment terminations or withdrawals. One serious adverse event was reported, which was not related to ILB® and resolved without sequalae. 270 mild/moderate adverse events were reported with no intolerable events occurring during the trial. The total number of ILB® treatments administered per patient ranged from 4 to 38, with a cumulative dose ranging from 745 to 6668 mg. As a result of the COVID-19 pandemic and the high-risk status of study participants, recruitment and treatment was suspended early in Mar-2020. At the long-term follow-up, three patients had died after the trial was halted, between 53 and 62 weeks after their final ILB® injection.
Interpretation: Long-term weekly ILB® injections of 2 mg/kg was well tolerated and had an acceptable safety profile in patients with ALS.
Trial registration: EudraCT: 2018-000668-28. clinicaltrials.gov: NCT03705390. This trial adheres to the principles of GCP in the design, conduct, recording and reporting of clinical trials as listed in part 2, "Conditions and Principles which apply to all Clinical Trials" under the header "Principles based on Articles 2 to 5 of the EU GCP Directive" in the Medicines for Human Use Clinical Trials Regulations (as amended in SI 2006/1928). For clarity, the study did not conform to all aspects of the International Conference on Harmonisation (ICH) E6 R2 Guidelines for GCP (also known as 'ICH GCP'). Of note, we did not use an external database, perform 100% source data verification, and only primary outcome data were analysed in parallel by a second, independent statistician.
Copyright: © 2024 Srinivasan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The consultancy work declared for AL and ZN relates to advice unrelated to this clinical trial. This clinical trial was undertaken independently with academic leads (VS, SPB), via the Drugs, Devices, Diagnostics and Biomarkers Team (D3B) at Birmingham’s Cancer Research UK Clinical Trials Unit and sponsored by the University of Birmingham. The funder (Tikomed AB) provided support in the form of salary contribution for authors (VS, VH, DB, ACJ, SJ, CP, KB, DS, AL, ZN, and SPB) as part of the clinical trial funding, but did not have any additional role, either direct or indirect, in the clinical trial study design, data collection, data analysis, decision to publish, or preparation of the manuscript through the participation of the co-authors. This does not alter our adherence to PLOS-ONE policies on sharing data and materials.
Figures
Similar articles
-
A phase II open label clinical study of the safety, tolerability and efficacy of ILB® for Amyotrophic Lateral Sclerosis.PLoS One. 2022 May 25;17(5):e0267183. doi: 10.1371/journal.pone.0267183. eCollection 2022. PLoS One. 2022. PMID: 35613082 Free PMC article. Clinical Trial.
-
Ropinirole hydrochloride remedy for amyotrophic lateral sclerosis - Protocol for a randomized, double-blind, placebo-controlled, single-center, and open-label continuation phase I/IIa clinical trial (ROPALS trial).Regen Ther. 2019 Jul 26;11:143-166. doi: 10.1016/j.reth.2019.07.002. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31384636 Free PMC article.
-
Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial.Lancet Neurol. 2017 Jul;16(7):505-512. doi: 10.1016/S1474-4422(17)30115-1. Epub 2017 May 15. Lancet Neurol. 2017. PMID: 28522181 Clinical Trial.
-
Cell-based therapies for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD011742. doi: 10.1002/14651858.CD011742.pub3. Cochrane Database Syst Rev. 2019. PMID: 31853962 Free PMC article.
-
Edaravone Oral Suspension: A Neuroprotective Agent to Treat Amyotrophic Lateral Sclerosis.Am J Ther. 2024 May-Jun 01;31(3):e258-e267. doi: 10.1097/MJT.0000000000001742. Am J Ther. 2024. PMID: 38691665 Review.
Cited by
-
The Potential of cfDNA as Biomarker: Opportunities and Challenges for Neurodegenerative Diseases.J Mol Neurosci. 2025 Mar 13;75(1):34. doi: 10.1007/s12031-025-02317-8. J Mol Neurosci. 2025. PMID: 40080233 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

