Point mutations at specific sites of the nsp12-nsp8 interface dramatically affect the RNA polymerization activity of SARS-CoV-2
- PMID: 38990941
- PMCID: PMC11260105
- DOI: 10.1073/pnas.2317977121
Point mutations at specific sites of the nsp12-nsp8 interface dramatically affect the RNA polymerization activity of SARS-CoV-2
Abstract
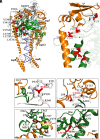
In a recent characterization of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) variability present in 30 diagnostic samples from patients of the first COVID-19 pandemic wave, 41 amino acid substitutions were documented in the RNA-dependent RNA polymerase (RdRp) nsp12. Eight substitutions were selected in this work to determine whether they had an impact on the RdRp activity of the SARS-CoV-2 nsp12-nsp8-nsp7 replication complex. Three of these substitutions were found around the polymerase central cavity, in the template entry channel (D499G and M668V), and within the motif B (V560A), and they showed polymerization rates similar to the wild type RdRp. The remaining five mutations (P323L, L372F, L372P, V373A, and L527H) were placed near the nsp12-nsp8F contact surface; residues L372, V373, and L527 participated in a large hydrophobic cluster involving contacts between two helices in the nsp12 fingers and the long α-helix of nsp8F. The presence of any of these five amino acid substitutions resulted in important alterations in the RNA polymerization activity. Comparative primer elongation assays showed different behavior depending on the hydrophobicity of their side chains. The substitution of L by the bulkier F side chain at position 372 slightly promoted RdRp activity. However, this activity was dramatically reduced with the L372P, and L527H mutations, and to a lesser extent with V373A, all of which weaken the hydrophobic interactions within the cluster. Additional mutations, specifically designed to disrupt the nsp12-nsp8F interactions (nsp12-V330S, nsp12-V341S, and nsp8-R111A/D112A), also resulted in an impaired RdRp activity, further illustrating the importance of this contact interface in the regulation of RNA synthesis.
Keywords: RNA synthesis; RNA-dependent RNA polymerase; nsp12–nsp8–nsp7 complex; primer extension; protein–protein interactions.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
MeSH terms
Substances
Grants and funding
- PID2020-117976 GB-I00/Ministerio de Ciencia e Innovación (MCIN)
- PID2020-113888RB-I00/Ministerio de Ciencia e Innovación (MCIN)
- 202220I116/Ministerio de Ciencia e Innovación (MCIN)
- PI21/00139/MEC | Instituto de Salud Carlos III (ISCIII)
- CSIC-COV19-014/MEC | Consejo Superior de Investigaciones Científicas (CSIC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

