TRPV1 and mast cell involvement in repeated variate stress-induced urinary bladder dysfunction in adult female mice
- PMID: 38991005
- PMCID: PMC11460343
- DOI: 10.1152/ajprenal.00125.2024
TRPV1 and mast cell involvement in repeated variate stress-induced urinary bladder dysfunction in adult female mice
Abstract
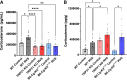
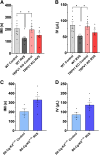
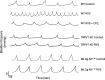
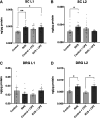
The etiology of interstitial cystitis/bladder pain syndrome (IC/BPS) is unknown but likely multifactorial. IC/BPS symptoms can be exacerbated by psychological stress, but underlying mechanisms remain to be defined. Transient receptor potential vanilloid 1 (TRPV1) channels, expressed on nerve fibers, have been implicated in bladder dysfunction and colonic hypersensitivity with stress in rodents. Histamine/H1R activation of TRPV1+ nerves increases bladder afferent fiber sensitivity to distension. TRPV1 channels are also expressed on mast cells, previously implicated in contributing to IC/BPS etiology and symptoms. We have examined the contribution of TRPV1 and mast cells to bladder dysfunction after repeated variate stress (RVS). RVS increased (P ≤ 0.05) serum and fecal corticosterone expression and induced anxiety-like behavior in wild-type (WT) mice. Intravesical instillation of the selective TRPV1 antagonist capsazepine (CPZ) rescued RVS-induced bladder dysfunction in WT mice. Trpv1 knockout (KO) mice did not increase voiding frequency with RVS and did not exhibit increased serum corticosterone expression despite exhibiting anxiety-like behavior. Mast cell-deficient mice (B6.Cg-Kitw-sh) failed to demonstrate RVS-induced increased voiding frequency or serum corticosterone expression, whereas control (no stress) mast cell-deficient mice had similar functional bladder capacity to WT mice. TRPV1 protein expression was significantly increased in the rostral lumbar (L1-L2) spinal cord and dorsal root ganglia (DRG) in WT mice exposed to RVS, but no changes were observed in lumbosacral (L6-S1) spinal segments or DRG. These studies demonstrated TRPV1 and mast cell involvement in RVS-induced increased voiding frequency and suggest that TRPV1 and mast cells may be useful targets to mitigate stress-induced urinary bladder dysfunction.NEW & NOTEWORTHY Using pharmacological tools and transgenic mice in a repeated variate stress (RVS) model in female mice, we demonstrate that transient receptor potential vanilloid 1 (TRPV1) and mast cells contribute to the increased voiding frequency observed following RVS. TRPV1 and mast cells should continue to be considered as targets to improve bladder function in stress-induced bladder dysfunction.
Keywords: corticosterone; cystometry; dorsal root ganglia; histamine; open-field test.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Mihaljevic M, Zeljic K, Soldatovic I, Andric S, Mirjanic T, Richards A, Mantripragada K, Pekmezovic T, Novakovic I, Maric NP. The emerging role of the FKBP5 gene polymorphisms in vulnerability-stress model of schizophrenia: further evidence from a Serbian population. Eur Arch Psychiatry Clin Neurosci 267: 527–539, 2016. doi: 10.1007/s00406-016-0720-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

