Contribution of Noncovalent Recognition and Reactivity to the Optimization of Covalent Inhibitors: A Case Study on KRasG12C
- PMID: 38991015
- PMCID: PMC11334105
- DOI: 10.1021/acschembio.4c00217
Contribution of Noncovalent Recognition and Reactivity to the Optimization of Covalent Inhibitors: A Case Study on KRasG12C
Abstract
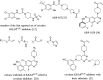
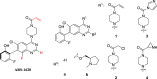
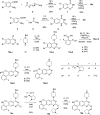
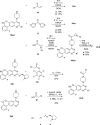
Covalent drugs might bear electrophiles to chemically modify their targets and have the potential to target previously undruggable proteins with high potency. Covalent binding of drug-size molecules includes a noncovalent recognition provided by secondary interactions and a chemical reaction leading to covalent complex formation. Optimization of their covalent mechanism of action should involve both types of interactions. Noncovalent and covalent binding steps can be characterized by an equilibrium dissociation constant (KI) and a reaction rate constant (kinact), respectively, and they are affected by both the warhead and the scaffold of the ligand. The relative contribution of these two steps was investigated on a prototypic drug target KRASG12C, an oncogenic mutant of KRAS. We used a synthetically more accessible nonchiral core derived from ARS-1620 that was equipped with four different warheads and a previously described KRAS-specific basic side chain. Combining these structural changes, we have synthesized novel covalent KRASG12C inhibitors and tested their binding and biological effect on KRASG12C by various biophysical and biochemical assays. These data allowed us to dissect the effect of scaffold and warhead on the noncovalent and covalent binding event. Our results revealed that the atropisomeric core of ARS-1620 is not indispensable for KRASG12C inhibition, the basic side chain has little effect on either binding step, and warheads affect the covalent reactivity but not the noncovalent binding. This type of analysis helps identify structural determinants of efficient covalent inhibition and may find use in the design of covalent agents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Copeland R. A.Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists, 2nd edition; John Wiley & Sons, 2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

