Targeting Myeloperoxidase Ameliorates Gouty Arthritis: A Virtual Screening Success Story
- PMID: 38991154
- PMCID: PMC11284790
- DOI: 10.1021/acs.jmedchem.4c00721
Targeting Myeloperoxidase Ameliorates Gouty Arthritis: A Virtual Screening Success Story
Abstract
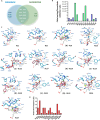
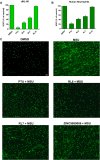
This study presents a new approach for identifying myeloperoxidase (MPO) inhibitors with strong in vivo efficacy. By combining inhibitor-like rules and structure-based virtual screening, the pipeline achieved a 70% success rate in discovering diverse, nanomolar-potency reversible inhibitors and hypochlorous acid (HOCl) scavengers. Mechanistic analysis identified RL6 as a genuine MPO inhibitor and RL7 as a potent HOCl scavenger. Both compounds effectively suppressed HOCl production in cells and neutrophils, with RL6 showing a superior inhibition of neutrophil extracellular trap release (NETosis). In a gout arthritis mouse model, intraperitoneal RL6 administration reduced edema, peroxidase activity, and IL-1β levels. RL6 also exhibited oral bioavailability, significantly reducing paw edema when administered orally. This study highlights the efficacy of integrating diverse screening methods to enhance virtual screening success, validating the anti-inflammatory potential of potent inhibitors, and advancing the MPO inhibitor research.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Cieza A.; Causey K.; Kamenov K.; Hanson S. W.; Chatterji S.; Vos T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396 (10267), 2006–2017. 10.1016/S0140-6736(20)32340-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

