Germ line ERG haploinsufficiency defines a new syndrome with cytopenia and hematological malignancy predisposition
- PMID: 38991192
- PMCID: PMC11530364
- DOI: 10.1182/blood.2024024607
Germ line ERG haploinsufficiency defines a new syndrome with cytopenia and hematological malignancy predisposition
Abstract
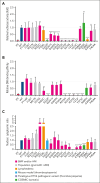
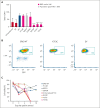
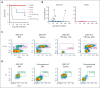
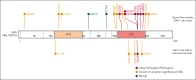
The genomics era has facilitated the discovery of new genes that predispose individuals to bone marrow failure (BMF) and hematological malignancy (HM). We report the discovery of ETS-related gene (ERG), a novel, autosomal dominant BMF/HM predisposition gene. ERG is a highly constrained transcription factor that is critical for definitive hematopoiesis, stem cell function, and platelet maintenance. ERG colocalizes with other transcription factors, including RUNX family transcription factor 1 (RUNX1) and GATA binding protein 2 (GATA2), on promoters or enhancers of genes that orchestrate hematopoiesis. We identified a rare heterozygous ERG missense variant in 3 individuals with thrombocytopenia from 1 family and 14 additional ERG variants in unrelated individuals with BMF/HM, including 2 de novo cases and 3 truncating variants. Phenotypes associated with pathogenic germ line ERG variants included cytopenias (thrombocytopenia, neutropenia, and pancytopenia) and HMs (acute myeloid leukemia, myelodysplastic syndrome, and acute lymphoblastic leukemia) with onset before 40 years. Twenty ERG variants (19 missense and 1 truncating), including 3 missense population variants, were functionally characterized. Thirteen potentially pathogenic erythroblast transformation specific (ETS) domain missense variants displayed loss-of-function (LOF) characteristics, thereby disrupting transcriptional transactivation, DNA binding, and/or nuclear localization. Selected variants overexpressed in mouse fetal liver cells failed to drive myeloid differentiation and cytokine-independent growth in culture and to promote acute erythroleukemia when transplanted into mice, concordant with these being LOF variants. Four individuals displayed somatic genetic rescue by copy neutral loss of heterozygosity. Identification of predisposing germ line ERG variants has clinical implications for patient and family diagnoses, counseling, surveillance, and treatment strategies, including selection of bone marrow donors and cell or gene therapy.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.C. is an employee of and stockholder in Illumina, Inc. The remaining authors declare no competing financial interests.
A complete list of the members of the ERG Variants Research Network appears in the supplemental Appendix.
Figures




Comment in
-
ERG: the good, the bad, and the ugly.Blood. 2024 Oct 24;144(17):1755-1756. doi: 10.1182/blood.2024025898. Blood. 2024. PMID: 39446373 No abstract available.
References
-
- Wilson NK, Foster SD, Wang X, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7(4):532–544. - PubMed
-
- Thoms JAI, Truong P, Subramanian S, et al. Disruption of a GATA2-TAL1-ERG regulatory circuit promotes erythroid transition in healthy and leukemic stem cells. Blood. 2021;138(16):1441–1455. - PubMed
-
- Loughran SJ, Kruse EA, Hacking DF, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9(7):810–819. - PubMed
-
- Diffner E, Beck D, Gudgin E, et al. Activity of a heptad of transcription factors is associated with stem cell programs and clinical outcome in acute myeloid leukemia. Blood. 2013;121(12):2289–2300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

