Endoplasmic reticulum exit sites are segregated for secretion based on cargo size
- PMID: 38991587
- PMCID: PMC11813558
- DOI: 10.1016/j.devcel.2024.06.009
Endoplasmic reticulum exit sites are segregated for secretion based on cargo size
Abstract
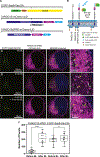
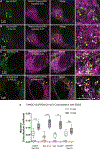
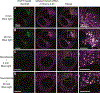
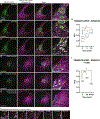
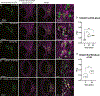
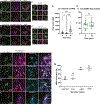
TANGO1, TANGO1-Short, and cTAGE5 form stable complexes at the endoplasmic reticulum exit sites (ERES) to preferably export bulky cargoes. Their C-terminal proline-rich domain (PRD) binds Sec23A and affects COPII assembly. The PRD in TANGO1-Short was replaced with light-responsive domains to control its binding to Sec23A in U2OS cells (human osteosarcoma). TANGO1-ShortΔPRD was dispersed in the ER membrane but relocated rapidly, reversibly, to pre-existing ERES by binding to Sec23A upon light activation. Prolonged binding between the two, concentrated ERES in the juxtanuclear region, blocked cargo export and relocated ERGIC53 into the ER, minimally impacting the Golgi complex organization. Bulky collagen VII and endogenous collagen I were collected at less than 47% of the stalled ERES, whereas small cargo molecules were retained uniformly at almost all the ERES. We suggest that ERES are segregated to handle cargoes based on their size, permitting cells to traffic them simultaneously for optimal secretion.
Keywords: COPII; ER export; ERES; TANGO1; collagens; endoplasmic reticulum; membrane traffic; optogenetics; protein secretion; secretory cargo.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

