A range of 30-62% of functioning multiciliated airway cells is sufficient to maintain ciliary airway clearance
- PMID: 38991708
- PMCID: PMC11469306
- DOI: 10.1183/13993003.01441-2023
A range of 30-62% of functioning multiciliated airway cells is sufficient to maintain ciliary airway clearance
Abstract
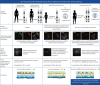
Background: Primary ciliary dyskinesia is a genetic disorder caused by aberrant motile cilia function that results in defective ciliary airway clearance and subsequently leads to recurrent airway infections and bronchiectasis. We aimed to determine: how many functional multiciliated airway cells are sufficient to maintain ciliary airway clearance?
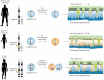
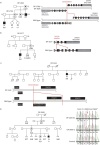
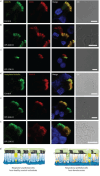
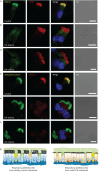
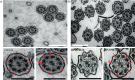
Methods: To answer this question we exploited the molecular defects of the X-linked recessive primary ciliary dyskinesia variant caused by pathogenic variants in DNAAF6 (PIH1D3), characterised by immotile cilia in affected males. We carefully analysed the clinical phenotype and molecular defect (using immunofluorescence and transmission electron microscopy) and performed in vitro studies (particle tracking in air-liquid interface cultures) and in vivo studies (radiolabelled tracer studies) to assess ciliary clearance of respiratory cells from female individuals with heterozygous and male individuals with hemizygous pathogenic DNAAF6 variants.
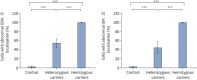
Results: Primary ciliary dyskinesia male individuals with hemizygous pathogenic DNAAF6 variants displayed exclusively immotile cilia, absence of ciliary clearance and severe primary ciliary dyskinesia symptoms. Owing to random or skewed X-chromosome inactivation in six female carriers with heterozygous pathogenic DNAAF6 variants, 54.3±10% (range 38-70%) of multiciliated cells were defective. Nevertheless, in vitro and in vivo assessment of the ciliary airway clearance was normal or slightly abnormal. Consistently, heterozygous female individuals showed no or only mild respiratory symptoms.
Conclusions: Our findings indicate that having 30-62% of multiciliated respiratory cells functioning can generate either normal or slightly reduced ciliary clearance. Because heterozygous female carriers displayed either no or subtle respiratory symptoms, complete correction of 30% of cells by precision medicine could improve ciliary airway clearance in individuals with primary ciliary dyskinesia, as well as clinical symptoms.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to declare.
Figures

Comment in
-
How many functioning ciliated airway epithelial cells are necessary for effective mucociliary clearance?Eur Respir J. 2024 Oct 10;64(4):2401573. doi: 10.1183/13993003.01573-2024. Print 2024 Oct. Eur Respir J. 2024. PMID: 39389615 No abstract available.
Similar articles
-
Skewed X-chromosome inactivation drives the proportion of DNAAF6-defective airway motile cilia and variable expressivity in primary ciliary dyskinesia.J Med Genet. 2024 May 21;61(6):595-604. doi: 10.1136/jmg-2023-109700. J Med Genet. 2024. PMID: 38408845
-
Pathogenic variants in CFAP46, CFAP54, CFAP74 and CFAP221 cause primary ciliary dyskinesia with a defective C1d projection of the central apparatus.Eur Respir J. 2024 Dec 12;64(6):2400790. doi: 10.1183/13993003.00790-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39362668 Free PMC article.
-
Cilia motility and structure in primary and secondary ciliary dyskinesia.Am J Rhinol Allergy. 2010 May-Jun;24(3):175-80. doi: 10.2500/ajra.2010.24.3448. Am J Rhinol Allergy. 2010. PMID: 20537282
-
Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome.Genet Med. 2009 Jul;11(7):473-87. doi: 10.1097/GIM.0b013e3181a53562. Genet Med. 2009. PMID: 19606528 Free PMC article. Review.
-
Advances in the Genetics of Primary Ciliary Dyskinesia: Clinical Implications.Chest. 2018 Sep;154(3):645-652. doi: 10.1016/j.chest.2018.05.007. Epub 2018 May 22. Chest. 2018. PMID: 29800551 Free PMC article. Review.
Cited by
-
International consensus statement on routine blood testing in primary ciliary dyskinesia.ERJ Open Res. 2025 Jun 23;11(3):01071-2024. doi: 10.1183/23120541.01071-2024. eCollection 2025 May. ERJ Open Res. 2025. PMID: 40551794 Free PMC article.
-
An integrated machine learning model of transcriptomic genes in multi-center chronic obstructive pulmonary disease reveals the causal role of TIMP4 in airway epithelial cell.Respir Res. 2025 Apr 23;26(1):158. doi: 10.1186/s12931-025-03238-1. Respir Res. 2025. PMID: 40269868 Free PMC article.
References
-
- Desai PB, Dean AB, Mitchell DR. Cytoplasmic preassembly and trafficking of axonemal dyneins. In: King SM, ed. Dyneins: Structure, Biology and Disease (Second Edition). London, Academic Press, 2018; pp. 140–161.
MeSH terms
LinkOut - more resources
Full Text Sources
