Organocatalytic asymmetric synthesis of Si-stereogenic silacycles
- PMID: 38992000
- PMCID: PMC11239892
- DOI: 10.1038/s41467-024-49988-2
Organocatalytic asymmetric synthesis of Si-stereogenic silacycles
Abstract
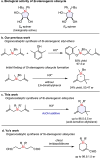
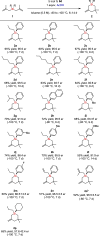
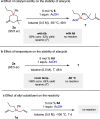
A strong and confined Brønsted acid catalyzed enantioselective cyclization of bis(methallyl)silanes provides enantioenriched Si-stereogenic silacycles. High enantioselectivities of up to 96.5:3.5 er were obtained for a range of bis(methallyl)silanes. NMR and ESI-MS studies reveal that the formation of a covalent adduct irreversibly inhibits turnover. Remarkably, we found that acetic acid as an additive promotes the collapse of this adduct, enabling full turnover. Experimental investigation and density functional theory (DFT) calculations were conducted to elucidate the origin of this phenomenon and the observed enantioselectivity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): a patent on the general catalyst class and its use in synthesis has been filed.
Figures


Similar articles
-
Catalytic asymmetric construction of 1,5-remote Si- and C-stereocenters via desymmetrizing ene reaction of bis(methallyl)silanes.Chem Sci. 2025 Apr 1;16(19):8454-8459. doi: 10.1039/d5sc01054c. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40225176 Free PMC article.
-
Organocatalytic Asymmetric Synthesis of Si-Stereogenic Silyl Ethers.J Am Chem Soc. 2022 Jun 15;144(23):10156-10161. doi: 10.1021/jacs.2c04261. Epub 2022 Jun 1. J Am Chem Soc. 2022. PMID: 35649270 Free PMC article.
-
Catalytic Asymmetric Construction of C- and Si-Stereogenic Silacyclopentanes via Hydrosilylation of Arylmethylenecyclopropanes.Angew Chem Int Ed Engl. 2024 Nov 25;63(48):e202413753. doi: 10.1002/anie.202413753. Epub 2024 Oct 17. Angew Chem Int Ed Engl. 2024. PMID: 39138131
-
An unusual autocatalysis with an air-stable Pd complex to promote enantioselective synthesis of Si-stereogenic enynes.Chem Sci. 2022 Dec 21;14(5):1123-1131. doi: 10.1039/d2sc06181c. eCollection 2023 Feb 1. Chem Sci. 2022. PMID: 36756338 Free PMC article.
-
Lewis Base-Catalyzed Dynamic Kinetic Asymmetric Transformation of Racemic Chlorosilanes en Route to Si-Stereogenic Silylethers.J Am Chem Soc. 2024 Aug 21;146(33):23092-23102. doi: 10.1021/jacs.4c04390. Epub 2024 Aug 6. J Am Chem Soc. 2024. PMID: 39108025
Cited by
-
Catalytic asymmetric construction of 1,5-remote Si- and C-stereocenters via desymmetrizing ene reaction of bis(methallyl)silanes.Chem Sci. 2025 Apr 1;16(19):8454-8459. doi: 10.1039/d5sc01054c. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40225176 Free PMC article.
-
Enantiopure Turbo Chirality Targets in Tri-Propeller Blades: Design, Asymmetric Synthesis, and Computational Analysis.Molecules. 2025 Jan 29;30(3):603. doi: 10.3390/molecules30030603. Molecules. 2025. PMID: 39942707 Free PMC article.
-
Phase-transfer-catalyst enabled enantioselective C-N coupling to access chiral boron-stereogenic BODIPYs.Nat Commun. 2025 Mar 20;16(1):2735. doi: 10.1038/s41467-025-58117-6. Nat Commun. 2025. PMID: 40108193 Free PMC article.
References
-
- Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry (Wiley, 2000).
-
- Auner, N. & Weis, J. Organosilicon Chemistry lll: From Molecules to Materials (Wiley, 2009).
-
- Hiyama, T. & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH, 2019).
LinkOut - more resources
Full Text Sources
Miscellaneous

