Harnessing virus flexibility to selectively capture and profile rare circulating target cells for precise cancer subtyping
- PMID: 38992001
- PMCID: PMC11239949
- DOI: 10.1038/s41467-024-50064-y
Harnessing virus flexibility to selectively capture and profile rare circulating target cells for precise cancer subtyping
Abstract
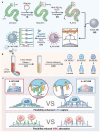
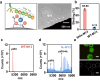
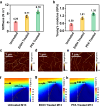
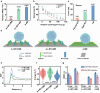
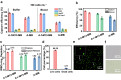
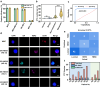
The effective isolation of rare target cells, such as circulating tumor cells, from whole blood is still challenging due to the lack of a capturing surface with strong target-binding affinity and non-target-cell resistance. Here we present a solution leveraging the flexibility of bacterial virus (phage) nanofibers with their sidewalls displaying target circulating tumor cell-specific aptamers and their ends tethered to magnetic beads. Such flexible phages, with low stiffness and Young's modulus, can twist and adapt to recognize the cell receptors, energetically enhancing target cell capturing and entropically discouraging non-target cells (white blood cells) adsorption. The magnetic beads with flexible phages can isolate and count target cells with significant increase in cell affinity and reduction in non-target cell absorption compared to magnetic beads having rigid phages. This differentiates breast cancer patients and healthy donors, with impressive area under the curve (0.991) at the optimal detection threshold (>4 target cells mL-1). Immunostaining of captured circulating tumor cells precisely determines breast cancer subtypes with a diagnostic accuracy of 91.07%. Our study reveals the power of viral mechanical attributes in designing surfaces with superior target binding and non-target anti-fouling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Target capturing performance of microfluidic channel surface immobilized aptamers: the effects of spacer lengths.Biomed Microdevices. 2019 Jun 15;21(3):54. doi: 10.1007/s10544-019-0403-z. Biomed Microdevices. 2019. PMID: 31203429
-
Fabrication of aptamer modified TiO2 nanofibers for specific capture of circulating tumor cells.Colloids Surf B Biointerfaces. 2020 Jul;191:110985. doi: 10.1016/j.colsurfb.2020.110985. Epub 2020 Mar 21. Colloids Surf B Biointerfaces. 2020. PMID: 32247218
-
Non-invasive isolation of rare circulating tumor cells with a DNA mimic of double-sided tape using multimeric aptamers.Nanoscale. 2019 Mar 28;11(13):5879-5883. doi: 10.1039/c9nr00364a. Nanoscale. 2019. PMID: 30869719
-
Aptamer-functionalized nano/micro-materials for clinical diagnosis: isolation, release and bioanalysis of circulating tumor cells.Integr Biol (Camb). 2017 Mar 1;9(3):188-205. doi: 10.1039/c6ib00239k. Epub 2017 Feb 1. Integr Biol (Camb). 2017. PMID: 28144664 Review.
-
Aptamer-Based Methods for Detection of Circulating Tumor Cells and Their Potential for Personalized Diagnostics.Adv Exp Med Biol. 2017;994:67-81. doi: 10.1007/978-3-319-55947-6_3. Adv Exp Med Biol. 2017. PMID: 28560668 Review.
Cited by
-
High-Viability Circulating Tumor Cells Sorting From Whole Blood at Single Cell Level Using Laser-Induced Forward Transfer-Assisted Microfiltration.Adv Sci (Weinh). 2025 May;12(18):e2414195. doi: 10.1002/advs.202414195. Epub 2025 Jan 27. Adv Sci (Weinh). 2025. PMID: 39868845 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

