SARS-CoV-2 serosurvey across multiple waves of the COVID-19 pandemic in New York City between 2020-2023
- PMID: 38992013
- PMCID: PMC11239669
- DOI: 10.1038/s41467-024-50052-2
SARS-CoV-2 serosurvey across multiple waves of the COVID-19 pandemic in New York City between 2020-2023
Abstract
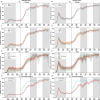
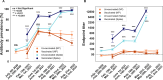
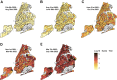
Sero-monitoring provides context to the epidemiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and changes in population immunity following vaccine introduction. Here, we describe results of a cross-sectional hospital-based study of anti-spike seroprevalence in New York City (NYC) from February 2020 to July 2022, and a follow-up period from August 2023 to October 2023. Samples from 55,092 individuals, spanning five epidemiological waves were analyzed. Prevalence ratios (PR) were obtained using Poisson regression. Anti-spike antibody levels increased gradually over the first two waves, with a sharp increase during the 3rd wave coinciding with SARS-CoV-2 vaccination in NYC resulting in seroprevalence levels >90% by July 2022. Our data provide insights into the dynamic changes in immunity occurring in a large and diverse metropolitan community faced with a new viral pathogen and reflects the patterns of antibody responses as the pandemic transitions into an endemic stage.
© 2024. The Author(s).
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines which list Florian Krammer as co-inventor. Dr. Simon is listed on the SARS-CoV-2 serological assays patent. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. Dr. Krammer has consulted for Merck, Seqirus, CureVac, GSK and Pfizer in the past and is currently consulting for Gritstone Bio, 3rd Rock Ventures, and Avimex, and he is a co-founder and scientific advisory board member of CastleVax. The Krammer laboratory has been collaborating with Pfizer on animal models for SARS-CoV-2. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

