Targeting soluble amyloid-beta oligomers with a novel nanobody
- PMID: 38992064
- PMCID: PMC11239946
- DOI: 10.1038/s41598-024-66970-6
Targeting soluble amyloid-beta oligomers with a novel nanobody
Abstract
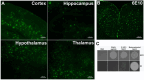
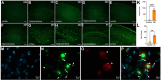
The classical amyloid cascade hypothesis postulates that the aggregation of amyloid plaques and the accumulation of intracellular hyperphosphorylated Tau tangles, together, lead to profound neuronal death. However, emerging research has demonstrated that soluble amyloid-β oligomers (SAβOs) accumulate early, prior to amyloid plaque formation. SAβOs induce memory impairment and disrupt cognitive function independent of amyloid-β plaques, and even in the absence of plaque formation. This work describes the development and characterization of a novel anti-SAβO (E3) nanobody generated from an alpaca immunized with SAβO. In-vitro assays and in-vivo studies using 5XFAD mice indicate that the fluorescein (FAM)-labeled E3 nanobody recognizes both SAβOs and amyloid-β plaques. The E3 nanobody traverses across the blood-brain barrier and binds to amyloid species in the brain of 5XFAD mice. Imaging of mouse brains reveals that SAβO and amyloid-β plaques are not only different in size, shape, and morphology, but also have a distinct spatial distribution in the brain. SAβOs are associated with neurons, while amyloid plaques reside in the extracellular matrix. The results of this study demonstrate that the SAβO nanobody can serve as a diagnostic agent with potential theragnostic applications in Alzheimer's disease.
© 2024. The Author(s).
Conflict of interest statement
BEW and BWS are co-founders of Turkey Creek Biotechnology LLC and have equity ownership. The authors declare no other competing conflicts of interest with the contents of this articles.
Figures




Update of
-
TARGETING SOLUBLE AMYLOID-BETA OLIGOMERS WITH A NOVEL NANOBODY.Res Sq [Preprint]. 2024 Mar 15:rs.3.rs-3944211. doi: 10.21203/rs.3.rs-3944211/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Jul 12;14(1):16086. doi: 10.1038/s41598-024-66970-6. PMID: 38559050 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

