Synergistic induction of mitotic pyroptosis and tumor remission by inhibiting proteasome and WEE family kinases
- PMID: 38992067
- PMCID: PMC11239683
- DOI: 10.1038/s41392-024-01896-z
Synergistic induction of mitotic pyroptosis and tumor remission by inhibiting proteasome and WEE family kinases
Abstract
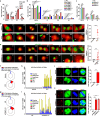
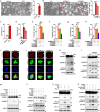
Mitotic catastrophe (MC), which occurs under dysregulated mitosis, represents a fascinating tactic to specifically eradicate tumor cells. Whether pyroptosis can be a death form of MC remains unknown. Proteasome-mediated protein degradation is crucial for M-phase. Bortezomib (BTZ), which inhibits the 20S catalytic particle of proteasome, is approved to treat multiple myeloma and mantle cell lymphoma, but not solid tumors due to primary resistance. To date, whether and how proteasome inhibitor affected the fates of cells in M-phase remains unexplored. Here, we show that BTZ treatment, or silencing of PSMC5, a subunit of 19S regulatory particle of proteasome, causes G2- and M-phase arrest, multi-polar spindle formation, and consequent caspase-3/GSDME-mediated pyroptosis in M-phase (designated as mitotic pyroptosis). Further investigations reveal that inhibitor of WEE1/PKMYT1 (PD0166285), but not inhibitor of ATR, CHK1 or CHK2, abrogates the BTZ-induced G2-phase arrest, thus exacerbates the BTZ-induced mitotic arrest and pyroptosis. Combined BTZ and PD0166285 treatment (named BP-Combo) selectively kills various types of solid tumor cells, and significantly lessens the IC50 of both BTZ and PD0166285 compared to BTZ or PD0166285 monotreatment. Studies using various mouse models show that BP-Combo has much stronger inhibition on tumor growth and metastasis than BTZ or PD0166285 monotreatment, and no obvious toxicity is observed in BP-Combo-treated mice. These findings disclose the effect of proteasome inhibitors in inducing pyroptosis in M-phase, characterize pyroptosis as a new death form of mitotic catastrophe, and identify dual inhibition of proteasome and WEE family kinases as a promising anti-cancer strategy to selectively kill solid tumor cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Phosphorylated IRF3 promotes GSDME-mediated pyroptosis through RIPK1/FADD/caspase-8 complex formation during mitotic arrest in ovarian cancer.Cell Commun Signal. 2025 Jul 1;23(1):306. doi: 10.1186/s12964-025-02322-9. Cell Commun Signal. 2025. PMID: 40597187 Free PMC article.
-
Antitumor effect of BC12-3 on multiple myeloma via proteasome inhibition.Med Oncol. 2025 Jun 3;42(7):235. doi: 10.1007/s12032-025-02804-3. Med Oncol. 2025. PMID: 40461880
-
Endothelin-1 (ET-1) induces resistance to bortezomib in human multiple myeloma cells via a pathway involving the ETB receptor and upregulation of proteasomal activity.J Cancer Res Clin Oncol. 2016 Oct;142(10):2141-58. doi: 10.1007/s00432-016-2216-2. Epub 2016 Aug 16. J Cancer Res Clin Oncol. 2016. PMID: 27530445 Free PMC article.
-
Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update.Int J Mol Sci. 2025 Jun 13;26(12):5701. doi: 10.3390/ijms26125701. Int J Mol Sci. 2025. PMID: 40565165 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Wee1 inhibitor PD0166285 sensitized TP53 mutant lung squamous cell carcinoma to cisplatin via STAT1.Cancer Cell Int. 2024 Sep 13;24(1):315. doi: 10.1186/s12935-024-03489-w. Cancer Cell Int. 2024. PMID: 39272147 Free PMC article.
-
Targeted delivery of the PKMYT1 inhibitor RP-6306 mediates PANoptosis in pancreatic cancer via mitotic catastrophe.Cell Death Dis. 2025 Jul 15;16(1):526. doi: 10.1038/s41419-025-07835-2. Cell Death Dis. 2025. PMID: 40664640 Free PMC article.
-
Saikosaponins Targeting Programmed Cell Death as Anticancer Agents: Mechanisms and Future Perspectives.Drug Des Devel Ther. 2024 Aug 21;18:3697-3714. doi: 10.2147/DDDT.S470455. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39185081 Free PMC article. Review.
-
Mechanistic insights into proteasome inhibitor MG132 induced apoptosis in melanoma A375 cells.Sci Rep. 2025 Apr 27;15(1):14731. doi: 10.1038/s41598-025-99151-0. Sci Rep. 2025. PMID: 40289224 Free PMC article.
-
Gaudichaudione H Enhances the Sensitivity of Hepatocellular Carcinoma Cells to Disulfidptosis via Regulating NRF2-SLC7A11 Signaling Pathway.Adv Sci (Weinh). 2025 Mar;12(11):e2411131. doi: 10.1002/advs.202411131. Epub 2025 Jan 22. Adv Sci (Weinh). 2025. PMID: 39840687 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

